Duration of antimicrobial treatment for complicated intra-abdominal infections after definitive source control: A systematic review, meta-analysis, and practice management guideline from the Eastern Association for the Surgery of Trauma
Published 2023
Citation: J Trauma. 95(4):p 603-612, October 2023
Authors
Ra, Jin H. MD; Rattan, Rishi MD; Patel, Nimitt J. MD; Bhattacharya, Bishwajit MD; Butts, Christopher A. PhD, DO; Gupta, Shailvi MD, MPH; Asfaw, Sofya H. MD; Como, John J. MD, MPH; Sahr, Sheryl M. MD, MS; Bugaev, Nikolay MD
Level of Evidence
Systematic Review and Meta-Analysis; Level III.
Complicated intra-abdominal infections (cIAIs) are a major cause of morbidity and mortality worldwide.[1] They are defined as infectious processes that extend beyond the affected organ and causes either localized or diffuse peritonitis.[1] Many pathological processes, such as acute appendicitis, acute diverticulitis, perforated peptic ulcers, perforated colon cancers, and infectious colitis, are included into this group. One important aspect of the clinical management of these conditions is the duration of antimicrobial treatment once definitive source control has been achieved. Definitive source control is defined as a procedure (surgical intervention or percutaneous drainage) to remove the infected fluid and/or tissue and to prevent further infection and contamination.[2][3] A commonly accepted practice has been to continue the antibiotic course for at least 7 days after definitive source control until sepsis is resolved.[2] Recently, the STOP-IT Trial by Sawyer et al.,[2] published in 2015, found no difference in clinical outcomes between patients who were treated with a fixed antibiotic course of 4 days versus treatment of antibiotics for 2 days after the resolution of symptoms with a median found to be 8 days. Guidelines from the World Society of Emergency Surgery (WSES), the Infectious Disease Society of America (IDSA), and the Surgical Infection Society all recommend a short course (fewer than 5 days) of antibiotics in those patients with cIAI and definitive source control.[1][3]
In the recent years, studies investigating outcomes associated with duration of antibiotics treatment in complicated intra-abdominal infection, have emerged. In light of this newly published data, we aimed to revisit and incorporate the data from those studies into the recommendations of WSES, IDSA and the Surgical Infection Society, as well as to apply a different methodology into making recommendations. A group composed of members of the Eastern Association for the Surgery of Trauma (EAST) was tasked with reviewing the current literature and formulating practice management guidelines for duration of antimicrobial treatment in cIAI after attaining definitive source control. EAST members published the organization's approach to practice management guideline development using Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology in 2012.[4] GRADE was created to provide a transparent method for developing and presenting summaries of evidence through a systemic approach for making clinical practice guideline recommendations. It has been widely adopted and is the preferred tool for grading the quality of evidence and for making recommendations in health care settings.[5]
Methods
The AGREE Reporting Checklist guideline was used to ensure proper reporting of methods, results, and discussion (SDC 1, https://links.lww.com/TA/D112).
The objective of this guideline was to determine the optimal duration of antibiotic treatment in adult patients who have undergone definitive source control of cIAI. The working group, consisting of EAST members, was assembled to conduct a systematic review, perform meta-analyses, and using the GRADE framework, formulate clinical recommendations pertaining to this topic. The population (P), intervention (I), comparator (C), and outcome (O) question was defined as follows:
PICO: In adult patients with complicated intra-abdominal infections who have undergone definitive source control (P), should a short (I) vs. long duration of antibiotic treatment (C) be used to reduce the risk of surgical site infections, unplanned radiological or surgical interventions, hospital length of stay, readmissions, and mortality (O)?
Outcome Measures
Clinical outcomes that could potentially delineate the effect of a short course of antibiotics were independently proposed by all authors. The importance of the outcome measures was determined through the rating of each outcome by the working group members, and the mean scores for each outcome were calculated. Outcomes were rated from 1 to 9: a rating of 1 to 3 was deemed not important; 4 to 6 was deemed important; and 7 to 9 was deemed critical. Only the critical and important outcomes were selected for further analysis. Critical outcomes included mortality, readmissions, surgical site infection, recurrent/persistent abscess, unplanned interventional radiology (IR) and surgical intervention, and sepsis/septic shock, while one outcome was felt to be important—hospital length of stay (LOS). The sepsis/septic shock outcome was still included despite having a single study looking into that outcome. This inclusion is supported in the GRADEPro handbook, which states that important outcomes should be included in the evidence profile whether or not information about them is available.[6] Definitive source control is defined here as both operative and percutaneous procedures targeting the source of cIAI.
Determining Noninferiority
During the protocol design, the group decided that noninferior results of the meta-analyses would allow us to make recommendations favoring the intervention. “Noninferiority” was established based on a concept of “not worse” effect of the intervention over control.
The US Food and Drug Administration's document titled “Noninferiority Clinical Trials to Establish Effectiveness, Guidance for Industry” recommends establishing noninferiority margins based on historical data where the effect of the active control treatment has been established over placebo.[7] The calculation for the noninferiority margins in this situation is done under “constancy assumption,” which refers to a presence of sufficient similarities between historic and current noninferiority studies. These similarities pertain to patient populations, concomitant treatments, dose of active control and analytical approaches. The “constancy assumption” is not applicable when there has been a significant evolution in the disease definitions, diagnosis, and treatment.[7] Taking into consideration the FDA recommendations, we chose acute appendicitis as the best historical reference for cIAI, given that there had been substantial changes in diagnosis and overall management of this condition over the last 70 years. Studies comparing management of acute appendicitis with and without (historical control) antibiotics were used to define noninferiority margins for risk difference for the selected outcomes: mortality, wound infection, and hospital length of stay[8][9] (SDC Table 1, https://links.lww.com/TA/D113). Per the FDA recommendations, noninferiority was confirmed when short course antibiotics preserved at least 90% of the lower bound 95% confidence interval (CI) of the long course antibiotics effect (SDC Table 1, https://links.lww.com/TA/D113).
The rest of the selected outcomes, such as recurrent abscess, unplanned IR/surgery and readmissions, were not reported allowing the application of the concept of “constancy assumption.” For these outcomes, the members of the working group independently proposed clinically acceptable noninferiority margins for each of the selected outcomes. Median values of the proposed noninferiority margins were calculated and reflected a difference of rate (%) between pooled results for the intervention (short course antibiotics) and control (long course antibiotics) groups. These are the noninferiority margins: abscess, 5%; unplanned IR or surgery, 5%; readmission, 7.5%.
Identification of References
The citations search was performed by medical librarians. Databases included in the literature search and MeSH terms are presented in SDC 2 (https://links.lww.com/TA/D114). The process of selecting studies for the final systematic review involved, screening of titles and abstracts, followed by a screening of full texts of the selected manuscripts (SDC Fig. 1, https://links.lww.com/TA/D115). Included studies were those that evaluated adult patients with cIAI who underwent a definitive source control of cIAI and were treated with either short or long duration of antibiotics course. At every screening step, each citation was evaluated by two team members. All disagreements were resolved by two group members (J.R., N.B.). Randomized controlled trials, prospective studies, and retrospective reviews with balanced comparison groups were considered for inclusion. Case reports/series, review articles, retrospective reviews without comparison groups, meta-analyses, non-English language publications, and articles reporting pediatric populations (patients <16 years) were excluded.
There were six randomized controlled trials (RCTs). Given the small number of RCTs, the decision was made to include observational and retrospective studies that reported comparison groups. Since there is no universal definition of short and long courses of antibiotic therapy, we used each article's definition of short and long duration.
Data Extraction and Methodology
The data extraction from each selected citation was performed by two group members. The data were extracted into Microsoft Excel spreadsheets (Microsoft Corp, Redmond, WA). Meta-analysis was done using Review Manager (RevMan) (Version 5.3; The Cochrane Collaboration, Oxford, United Kingdom). The continuous variables, specifically hospital LOS, were reported as a mean difference (MD). For the dichotomous outcomes, odds ratios (OR) were calculated. Confidence intervals of 95% for MD and OR were provided with a declared statistical significance of p < 0.05. Random-effects modeling was used for both dichotomous and continuous variables.
Methodology Quality Assessment
The GRADE methodology was used to assess the quality of the selected studies, which were ranked as high, moderate, low, or very low. To ensure that the evidence demonstrated a precise estimate of effect, the following principles were also considered: risk of bias, inconsistency, indirectness, imprecision, and publication bias. Each of the principles was assessed and included in the evidence profile for each outcome. Based on this, the quality of evidence was graded up or down. Evidence tables were developed using the GRADEpro Guidelines Development Tool (Evidence Prime Inc., Hamilton, Ontario, Canada).
Recommendations were made based upon the established risk-benefit ratios and how they applied to accepted patient values and preferences, balance between benefits and harms, cost, and resource issues as well as the quality of evidence. The strength of the recommendation was determined by the group. A strong recommendation would be prefaced with the phrase “we recommend” whereas a weak recommendation would be prefaced with “we conditionally recommend.”
Measurement of Heterogeneity
To determine whether the study comparisons shared similar characteristics and that the patients were similar and received comparable care, the level of heterogeneity was evaluated using the RevMan software. The I[2] (%) statistic, or heterogeneity was calculated, and the degree of heterogeneity was reflected in the values obtained. The higher the value, the greater the degree of heterogeneity between patient populations. For example, an I[2] value of 25% to 49% reflected low heterogeneity, an I[2] value of 50% to 74% was moderate, and an I[2] value of 75% to 100% was considered high.[10] In addition, forest plots were generated to calculate effect estimates and associated CI for each of the selected outcomes.
Results
PICO: In adult patients with complicated intra-abdominal infections who have undergone definitive source control (P), should a short (I) versus long duration of antibiotic treatment (C) be used to reduce the risk of surgical site infections, unplanned radiological or surgical interventions, hospital length of stay, readmissions, and mortality (O)?
Qualitative Analysis
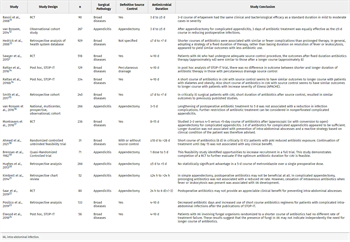
A total of 16 studies were included in this guideline for qualitative analysis. See Table 1 for details of all studies included. There were five RCTs,[2][11][17][18][22] one quasi-randomized clinical trial[19] (this study postoperatively randomized treatment arms), two observational cohort studies[12][16] and five retrospective studies with comparison group.[13][15][20][21][23] The data from Sawyer et al. Randomized controlled trial underwent post hoc analyses and were published in three additional manuscripts.[14][24][25] Those post hoc studies were not included in the quantitative analysis and one of the three studies were excluded from qualitative analysis as it looked at fungal IAI specifically.[24] The sixteen studies showed a wide range of heterogeneity (0–100%) among them. The sample sizes varied from 31 patients to 929 patients. All studies included adults (≥16 years old), except one that included children and adults.[16] Two studies specifically looked at critically ill patients.[15][17] In defining what was considered source control for the included studies, we found the following: one study did not specify source control methods,[13] seven studies were focused on surgical control,[11][12][16][19–22] seven studies specifically included both operative and percutaneous methods,[2][14][15][17][18][23][24] and one study only focused on percutaneous procedure outcomes.[25]
For the purpose of this systematic review, the determination of a short or long duration of antimicrobial treatment was performed by taking the average of how short and long durations were defined in each of the included studies. The studies and the duration of the antimicrobial treatment are listed in Table 1, with the calculated average duration of the control and the experimental arms. A short duration ranged from one dose to ≤ 10 days with an average of 4 days and the long duration ranged from >1 day to 28 days with an average of 8 days. Even with the outliers for both the short and long duration removed, the short duration average was still 4 days and the long duration was 7.9 days.
Quantitative Analysis
There were seven initial outcomes that the task force had chosen: six critical (mortality, surgical site infection, persistent/recurrent abscess, unplanned interventional or operative interventions, sepsis and septic shock, and readmissions) and one important (hospital LOS). The outcome “sepsis and septic shock” was reported in only one study. This outcome was included based on the GRADE Pro Handbook recommendation that outcomes selected by the panel be included whether or not information is available for them.[6]
Mortality
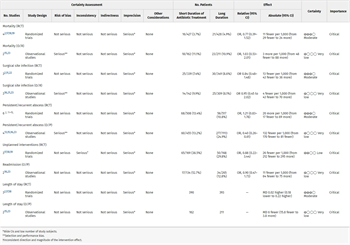
There were six studies that reported mortality, three of which were RCTs (Table 2; SDC Fig. 2A, https://links.lww.com/TA/D189). Taken separately, none of the included studies reported statistically significant difference between the short- and long-term antibiotics courses. The initial pooled analysis of those six studies showed no difference between a short and long duration of antibiotics (OR, 0.90; 95% CI, 0.56–1.44). The heterogeneity was 0% among the studies. The subgroup analyses of the RCTs and the observational and retrospective studies (O/R), did not demonstrate statistically significant difference between short and long duration for mortality (SDC Fig. 2A1 and Fig. 2A2, https://links.lww.com/TA/D189). Subgroup analysis performed by cohorting the studies into similar short and long treatment duration (4 days vs. 10 days and 8–10 days vs. 15–18 days) groups, showed no significant difference in mortality (SDC Fig. 3A1 and 3A2, https://links.lww.com/TA/D190).
Surgical Site Infection
There were six studies that looked at the surgical site infection outcome, three of which were RCTs (SDC Fig. 2B, https://links.lww.com/TA/D189, Table 2). Surgical site infections were reported as superficial,[21][22] deep,[22] and were not specified as either superficial or deep.[2][11][16][23] This outcome did not include organ specific infections or intra-abdominal abscesses.
The initial pooled analysis of the six included studies showed no difference between a short and long duration of antibiotics (OR, 0.88; 95% CI, 0.56–1.38) (SDC Fig. 2B, https://links.lww.com/TA/D189). Also, when separating the RCTs and the observational and retrospective (O/R) studies, there was no statistically significant difference between short and long duration for SSIs (SDC Fig. 2B1 and 2B2, https://links.lww.com/TA/D189). The heterogeneity among these studies was 0%. Subgroup analysis, cohorting the studies by similar short and long treatment duration (1 day vs. 1–6 days; 3 days vs. 5 days, 4 days vs. 10 days), failed to demonstrate statistical significance (SDC Fig. 3B1, 3B2 and 3B3, https://links.lww.com/TA/D190).
Persistent/Recurrent Abscess
Ten studies evaluated the outcome of persistent and recurrent abscess, and four of them were RCTs (SDC Fig. 2C, https://links.lww.com/TA/D189, Table 2). The initial pooled analysis of the 10 included studies showed no difference between short and long duration of antibiotics (OR, 0.76; 95% CI, 0.45–1.29) (SDC Fig. 2C, https://links.lww.com/TA/D190). There was high heterogeneity among the studies at 69%. When separating the RCTs and observation and retrospective (O/R) studies, there was no statistically significant difference between short and long duration for persistent and recurrent abscess among the RCTs (SDC Fig. 2C1, https://links.lww.com/TA/D190). However, there was a significant statistically difference among the observational and retrospective studies that favored short duration of antimicrobial treatment with some heterogeneity (I[2] = 33%) among the studies (Fig. 2C2). Subgroup analysis cohorting the studies by similar short and long treatment duration showed no statistical significance in 3 days versus 5 days; 4 days versus 10 days; 8 days to 10 days versus 15 days to 28 days (SDC Fig. 3C1, 3C3, and 3C4, https://links.lww.com/TA/D190). For the ≤7-day versus >7-day cohort, the abscess outcome favored shorter duration (SDC Fig. 3C2, https://links.lww.com/TA/D190).
Unplanned Interventional Radiology/Operative Intervention
Four studies looked at the outcome of unplanned interventional radiology/operative intervention, three of which were RCTs[17][18][22] and one observational[15] (SDC Fig. 2D, https://links.lww.com/TA/D189, Table 2). Brennan et al.,[19] who studied outcomes of complicated acute appendicitis patients, and Ahmed et al.,[18] who studied patients with complicated intra-abdominal infections, showed no statistically significant difference between short and long duration of antibiotics. Montravers et al.[17] and Smith et al.[15] reported management of complicated intra-abdominal infections in critically ill patients. Montravers et al.[17] demonstrated lower risk of reintervention in 15-day versus 8-day course. Smith et al.[15] reported lower risk of shorter antibiotics duration.
The initial pooled analysis of four included studies showed no difference between the short and long duration of treatment (OR, 0.53; 95% CI, 0.12–2.26). The subgroup analysis cohorting the RCTs showed no difference between short and long duration of antibiotics (OR, 0.88; 95% CI, 0.22–3.44) (SDC Fig. 2D1, https://links.lww.com/TA/D189). The heterogeneity among the studies was 89%. The subgroup analysis; cohorting the studies by similar short, 8 days to 10 days, versus long, 15 days to 28 days, treatment duration; demonstrated no statistically significant difference between the groups (SDC Fig. 3D1, https://links.lww.com/TA/D190).
Readmission
Three studies reported the readmission outcome, one of which was an RCT[22] and two observational[16][23] (SDC Fig. 2E, https://links.lww.com/TA/D189, Table 2). Saar et al.[22] and van Rossem et al.[12] studied the outcomes of patients with complicated appendicitis and Posillico et al.[23] studied outcomes in patients with complicated intra-abdominal infections. The initial pooled analysis including all three studies showed no statistical differences between short and long-term course of antibiotics (OR, 0.92; 95% CI, 0.50–1.69) (SDC Fig. 2E, https://links.lww.com/TA/D189). The heterogeneity among the studies was 0%. A subsequent pooled analysis cohorting the two observational studies showed no difference between short and long duration of antibiotics (OR, 0.90; 95% CI, 0.47–1.73) (SDC Fig. 2E1, https://links.lww.com/TA/D189). The heterogeneity among the studies was low at 0%.
Hospital Length of Stay
Five studies looked at hospital LOS, three of which were RCTs[2][17][18] and two were observational[15][23] (SDC Fig. 2F, https://links.lww.com/TA/D189, Table 2). The initial pooled analysis of the five studies showed no statistical difference between the short and long duration (OR, −2.62; 95% CI, −7.08 to 1.83). The heterogeneity was very high at 100%. The separate pooled analyses of RCT versus observational and retrospective studies showed no difference between short and long duration of antibiotics (SDC Fig. 2F1 and Fig. 2F2, https://links.lww.com/TA/D189). The heterogeneity among the observation and retrospective studies were 100%, and for RCTs the heterogeneity was 0%. Subgroup analysis cohorting the studies by similar short and long treatment duration showed no statistical significance between the 4-day and 10-day; 8- to 10-day and 15- to 28-day cohort (SDC Fig. 3E1 and Fig. 3E2, https://links.lww.com/TA/D190). The heterogeneity for the 4-day versus 10-day cohort was high at 96% and was low for the other cohort at 0%.
Sepsis/Septic Shock
One RCT reported sepsis and septic shock outcome[17] (SDC Fig. 2G, https://links.lww.com/TA/D189). Montravers et al.[17] studied antibiotic duration in critically ill intensive care unit (ICU) patients with postoperative intra-abdominal infections. Short duration was defined as 8 days and long duration was defined as 15 days. There was no clinical benefit for the longer vs. shorter duration in critically ill ICU patients (OR, 2.69; 95% CI, 0.86–9.96; p = 0.06).
Grading of the Evidence
The quality of the evidence was assessed as low (Table 2). Although there were five randomized control trials and one quasi-randomized clinical trial, the level of evidence was downgraded for several reasons.
In addition to the six RCTs, there were five retrospective reviews, two observational studies and three post hoc analyses of a single RCT were included into this systematic review. The inclusion of non-RCTs downgraded the level of evidence due to limitations of study’s design. The publication bias was determined since only studies with positive results were published, and because there was asymmetry in the funnel plots (SDC Fig. 4, https://links.lww.com/TA/D118). Across all outcomes, a serious imprecision was detected due to a low number of included subjects and wide CIs. The heterogeneity ranged widely from 0% to 100%.
Recommendation
In adult patients with complicated intra-abdominal infections who have undergone definitive source control, we recommend a short (4 days) versus long (8 days) duration of antimicrobial treatment. The definition of definitive source control included both operative and percutaneous procedures. This recommendation is based on the noninferior effect of a short versus long antibiotic course duration, taking into account the lower risk of antibiotic related complications along with reduced cost.[22][26][27] Mortality and length of stay outcomes preserved 90% of historically established risk difference effect of “no antibiotics” versus “antibiotics.” Rates of recurrent abscess, unplanned IR/surgery and readmission were noninferior based on the working groups established noninferiority margins. The working group assumed that an average patient would prefer a shorter exposure to antibiotics. Antibiotic stewardship was created in an effort to reduce inappropriate antibiotic utilization, avoid increased cost of care, and decrease the incidence of adverse clinical events such as the emergence of multidrug-resistant (MDR) organisms and Clostridium difficile infections.[22][26][27] While the quality of evidence was low when looking at the studies as a whole, there were six randomized control trials including the STOP-IT trial which was a study with over 500 patients and was well designed. Also, it is unclear if there will be any further studies that will provide stronger evidence to support shorter duration of treatment. The GRADE Handbook states that the strength of the recommendation should be based on the confidence of the work group that the desirable effects of the intervention outweigh the undesirable effects. Our initial recommendation was that we conditionally recommended a shorter duration of antibiotics. This decision was based mostly on the quality of evidence rather than the impact or implications it would have on the clinicians and the patients. We decided to look more carefully into our GRADE methodology and decided that we needed to rethink about our recommendation. We performed a blind vote among the group after each member considered the evidence as well as reviewing the GRADE Handbook discussion about deciding the strength of recommendations. More than 70% of the group voted in favor of a stronger recommendation over the previous conditional recommendation.
In determining the generalizability of our recommendation. We recommend that most patients, including those who are critically ill and/or have multiple comorbid conditions would benefit from a shorter duration of antibiotics. There were three studies that specifically focused on critically ill or high Acute Physiology and Chronic Health Evaluation (APACHE) score populations with similar findings between the long and short duration of treatment.[14][15][18] Ahmed et al.[18] was a feasibility randomized control trial of 31 patients. Rattan et al.[14] was as post hoc analysis of the STOP-IT trial looking specifically at diabetic and obese patients as well as those with high APACHE scores with 334 patients. Smith et al.[15] was a retrospective study of 240 patients. Therefore, this recommendation would include those with multiple comorbid conditions or critically ill.
Using This Guideline in Clinical Practice
This guideline represents the results of a systematic review of the available evidence regarding antimicrobial treatment duration in adult patients with complicated intra-abdominal infections after definitive source control. Patients with complicated intra-abdominal infections should undergo initial fluid resuscitation, timely initiation of empiric antimicrobial treatment and definitive source control.[28] Surgical removal or repair of the affected organ and an adequate drainage (surgical or percutaneous) serve as options of the definitive source control. Obtaining cultures of the infectious source should be a part of the initial management. Once definitive source control has been achieved, the recommendations from this practice management guideline can be applied. The noninferior effect and a low risk of antibiotic related complications, favors a short versus long duration of antibiotics. The exact antibiotic regimen should be considered according to the surgical pathology, individual patients characteristics and local institutional protocols. The recommendation for short duration (defined as four or less days of antimicrobial treatment) over long duration (defined as 8 days or longer) was made despite the overall low quality of the available evidence, the evidence included six randomized control trial including one that was a high-quality large study, the STOP-IT Trial[24] that will not likely be replicated in the near future. Although, there was a lack of universal definition of short or long duration of treatment, for the purpose of this guideline, we took the average of the short versus long duration from the studies included.
Other considerations were taken into account when making the recommendation for a shorter duration of antimicrobial treatment. Posillico et al.[23] found that a shorter antibiotic course was associated with median hospital cost, but this difference was not statistically significant. The study by Montravers et al.,[17] one of the three studies included in our meta-analysis, evaluated the length of therapy and emergence of multidrug resistant (MDR) organisms and found that there was no statistically significant difference between short (8 days) and long (15 days) treatment arms. Smith et al.,[15] the other study that evaluated for the emergence of MDR organisms, found that although there was a trend toward increased MDR resistance in patients who received a longer duration, this was not statistically significant. The third study was by Sawyer et al.,[2] and in this article, the authors found no statistically significant difference in C. difficile infection and extra-abdominal infection with resistant organisms between the two groups. The study by Rattan et al.[25] was the only study that compared C. difficile infections in the short and long durations groups and this group found no statistical difference between the two and recommended a larger study.
Subgroup analysis, cohorting the studies by similar treatment duration, did not show statistically significant difference between shorter vs. longer treatment duration except for the persistent and recurrent abscess outcome favoring shorter duration of ≤7 days. Additional subgroup analysis cohorting RCTs and observation and retrospective studies, did not statistically show significant differences between a short versus a long duration of antimicrobial treatment for each of the outcomes except for persistent and recurrent abscess. Among the observational and retrospective studies, shorter duration was favored which was those treated for ≤7 days. However, despite the differences in study design, quality of the studies, risk of bias, heterogeneity, and imprecision, our analysis showed that a shorter duration of antibiotic therapy was noninferior to a long duration of antimicrobial treatment. Therefore, there is no reason to subject a patient to a longer course of antibiotics. Potential benefits of a short duration of treatment are: decreasing the risk of antimicrobial resistance, side effects of antibiotics, decreased risk of C. difficile infections, as well as increased options for patient monitoring either in the inpatient or outpatient setting, and possibly an decreased cost of medical care.
There were several limitations of this practice management guideline. The majority of the included studies were observational. Six of the 16 studies specifically looked at treatment of appendicitis and whether these data can be applied to all complicated intra-abdominal infections in adults, may leave that question not fully answered until further high-quality studies are performed. One of the post hoc analyses of the STOP-IT trial[24] looked specifically at the duration of treatment in fungal intra-abdominal infections, and it is unclear if fungal infections require a similar duration of treatment as bacterial infections and thus it was removed from the quantitative analysis. Additional limitations of this systemic review include the risk of incomplete retrieval of identified references due to the inclusion only English language manuscripts.
Future Investigations
Further high-quality studies are needed to further support these recommendations and improve the level of evidence regarding the optimal duration of antimicrobial treatment for intra-abdominal infections in adults after definitive source control. Also, more specific definitions of short and long durations of treatment would help providers decide on the duration of treatment. Whether fungal intra-abdominal infections or those with resistant pathogens specifically need a different treatment duration is yet undetermined. Whether the immunocompromised or patients with comorbid conditions need a longer duration of antimicrobial treatment, further study is also necessary. Rattan et al.,[14] in post hoc analysis of the STOP-IT Trial[24] did look specifically at those patients with obesity, diabetes, or APACHE II Score of ≥15 and found that those patients treated with 4 days of antibiotics had similar outcomes to those treated for longer. Also, further work is needed to determine which patients may warrant a longer duration of treatment such as biomarkers for ongoing sepsis, treatment for resistant organisms, and those patients who are immunocompromised. Hedrick et al[13] did study whether shorter duration (≤7 days) of antibiotics was associated with similar outcomes compared with longer duration (>7 days) by looking at the difference between a fixed duration of treatment and one based on physiologic measures such as leukocytosis and fever. They found that shorter or fixed duration was associated with similar or fewer complications than prolonged therapy based on physiologic measures and recommended larger randomized trials.
In conclusion, in adults with intra-abdominal infection after definitive source control, our committee recommended a short duration, defined as 4 days or shorter, of antimicrobial treatment, over a long duration, defined as 8 days or longer. Considerations included a noninferior effect of a short duration of antibiotics on the selected outcomes, cost, patient preference for a short course of treatment, and potential harm of multidrug resistance, and drug interactions associated with the longer treatment.
Authorship
J.H.R. participated in the study idea, study design, literature search, data collection, data analysis, data interpretation, drafting the article, and critical revisions. R.R. participated in the study design, data collection, data interpretation, and critical revisions. N.J.P. participated in the study design, data collection, data interpretation, and critical revisions. B.B. participated in the study design, data collection, data interpretation, and critical revisions. C.A.B. participated in the study design, data collection, data interpretation, and critical revisions. S.G. participated in the study design, data collection, data interpretation, and critical revisions. S.H.A. participated in the study design, data collection, data interpretation, and critical revisions. J.J.C. participated in the study design, data collection, data interpretation, and critical revisions. S.M.S. participated in the study design, data collection, data interpretation, and critical revisions. N.B. participated in the study idea, study design, literature search, data collection, data analysis, data interpretation, drafting the article, and critical revisions.
Disclosure
The authors declare no funding or conflicts of interest.
References
- Sartelli M, Chichom-Mefire A, Labricciosa FM, Hardcastle T, Abu-Zidan FM, Adesunkanmi AK, et al. The Management of Intra-abdominal Infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;21:36.
- Sawyer RG, Claridge JA, Nathens AB, Rotstein OD, Duane TM, Evans HL, et al. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med. 2015;372(21):1996–2005.
- Mazuski JE, Tessier JM, May AK, Sawyer RG, Nadler EP, Rosengart MR, et al. The surgical infection society on the management of intra-abdominal infection. Surg Infect. 2017;18(1):1–76.
- Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, et al. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S283–S287.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926.
- Schunemann H, Brozek J, Guyatt G, Oxman A, eds. 3.4 Surrogate (substitute) outcomes. GRADE Handbook 2013. https://gdt.gradepro.org/app/handbook/. Accessed 09-10-2021.
- https://www.fda.gov/media/78504/download.
- Gilmour IE, Lowdon AG. Acute appendicitis. Edinb Med J. 1952;59(8):361–373.
- Hawk JC Jr., Becker WF, Lehman EP. Acute appendicitis. III. Analysis of 1003 cases. Ann Surg. 1950;132(4):729–745.
- Cochrane Handbook for Systematic Reviews of Interventions, chapter 10.10.2. https://training.cochrane.org/handbook/current/chapter-10. Accessed 04-04-2021.
- Basoli A, Chirletti P, Cirino E, D'Ovidio NG, Doglietto GB, Giglio D, et al. A prospective, double-blind, multicenter, randomized trial comparing ertapenem 3 vs >or=5 days in community-acquired intraabdominal infection. J Gastrointest Surg. 2008;12(3):592–600.
- van Rossem CC, Schreinemacher MH, Treskes K, van Hogezand RM, van Geloven AA. Duration of antibiotic treatment after appendicectomy for acute complicated appendicitis. Br J Surg. 2014;101(6):715–719.
- Hedrick TL, Evans HL, Smith RL, McElearney ST, Schulman AS, Chong TW, et al. Can we define the ideal duration of antibiotic therapy? Surg Infect. 2006;7(5):419–432.
- Rattan R, Allen CJ, Sawyer RG, Mazuski J, Duane TM, Askari R, et al. Patients with risk factors for complications do not require longer antimicrobial therapy for complicated intra-abdominal infection. Am Surg. 2016;82(9):860–866.
- Smith SE, Rumbaugh KA, May AK. Evaluation of a short course of antimicrobial therapy for complicated intra-abdominal infections in critically ill surgical patients. Surg Infect. 2017;18(6):742–750.
- van Rossem CC, Schreinemacher MH, van Geloven AA, Bemelman WA. Antibiotic duration after laparoscopic appendectomy for acute complicated appendicitis. JAMA Surg. 2016;151(4):323–329.
- Montravers P, Tubach F, Lescot T, Veber B, Esposito-Farese M, Seguin P, et al. Short-course antibiotic therapy for critically ill patients treated for postoperative intra-abdominal infection: the DURAPOP randomised clinical trial. Intensive Care Med. 2018;44(3):300–310.
- Ahmed S, Brown R, Pettinger R, Vargas-Palacios A, Burke D, Kirby A. The CABI Trial: an unblinded parallel group randomised controlled feasibility trial of long-course antibiotic therapy (28 days) compared with short course (≤ 10 days) in the prevention of relapse in adults treated for complicated intra-abdominal infection. J Gastrointest Surg. 2021;25:1045–1052.
- Brennan SS, Smith GM, Evans M, Pollock AV. The management of the perforated appendix: a controlled clinical trial. Br J Surg. 1982;69(9):510–512.
- Hughes MJ, Harrison E, Paterson-Brown S. Post-operative antibiotics after appendectomy and post-operative abscess development: a retrospective analysis. Surg Infect. 2013;14(1):56–61.
- Kimbrell AR, Novosel TJ, Collins JN, Weireter LJ, Terzian HW, Adams RT, et al. Do postoperative antibiotics prevent abscess formation in complicated appendicitis? Am Surg. 2014;80(9):878–883.
- Saar S, Mihnovitš V, Lustenberger T, Rauk M, Noor EH, Lipping E, et al. Twenty-four hour versus extended antibiotic administration after surgery in complicated appendicitis: a randomized controlled trial. J Trauma Acute Care Surg. 2019;86(1):36–42.
- Posillico SE, Young BT, Ladhani HA, Zosa BM, Claridge JA. Current evaluation of antibiotic usage in complicated intra-abdominal infection after the STOP IT trial: did we STOP IT? Surg Infect. 2019;20(3):184–191.
- Elwood NR, Guidry CA, Duane TM, Cuschieri J, Cook CH, O'Neill PJ, et al. Short-course antimicrobial therapy does not increase treatment failure rate in patients with intra-abdominal infection involving fungal organisms. Surg Infect. 2018;19(4):376–381.
- Rattan R, Allen CJ, Sawyer RG, Askari R, Banton KL, Coimbra R, et al. Percutaneously drained intra-abdominal infections do not require longer duration of antimicrobial therapy. J Trauma Acute Care Surg. 2016;81(1):108–113.
- Coakley BA, Sussman ES, Wolfson TS, Bhagavath AS, Choi JJ, Ranasinghe NE, et al. Postoperative antibiotics correlate with worse outcomes after appendectomy for nonperforated appendicitis. J Am Coll Surg. 2011;213(6):778–783.
- Dellit TH, Owens RC, McGowan JE Jr. Infectious Diseases Society of America. Guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177.
- Dellinger RP, Schorr CA, Levy MM. Users’ guide to the 2016 surviving sepsis guidelines. Crit Care Med. 2017;45(3):381–385.