Pancreatic Injuries
Published 2017
Citation: J Trauma. 82(1):185-199, January 2017
Authors
Ho, Vanessa Phillis MD, MPH; Patel, Nimitt J. MD; Bokhari, Faran MD; Madbak, Firas G. MD; Hambley, Jana E. MD; Yon, James R. MD; Robinson, Bryce R.H. MD; Nagy, Kimberly MD; Armen, Scott B. MD; Kingsley, Samuel MD; Gupta, Sameer MD; Starr, Frederic L. MD; Moore, Henry R. III MD; Oliphant, Uretz J. MD; Haut, Elliott R. MD, PhD; Como, John J. MD, MPH
Author Information
From the Division of Trauma, Department of Surgery (V.P.H., J.E.H.), Surgical Critical Care and Acute Care Surgery, University Hospitals Cleveland Medical Center; Division of Trauma, Department of Surgery (N.J.P., J.J.C.), Critical Care, Burns, and Acute Care Surgery, MetroHealth Medical Center, Cleveland, Ohio; Department of Trauma and Burn (F.B., K.N., S.G., F.L.S.), John H. Stroger, Jr. Hospital of Cook County, Chicago, Illinois; Department of Surgery (F.G.M.), University of Florida College of Medicine, Jacksonville, Florida; Department of Surgery (J.R.Y.), Swedish Medical Center, Englewood, Colorado; Division of Trauma and Burns, Department of Surgery (B.R.H.R.), Harborview Medical Center, University of Washington, Seattle, Washington; Division of Trauma, Department of Surgery (S.B.A.), Acute Care, & Critical Care Surgery, Penn State Hershey College of Medicine, Hershey, Pennsylvania; Department of Surgery (S.K.), Illinois Masonic Hospital, Chicago; Department of Surgery (H.R.M., U.J.O.), University of Illinois College of Medicine, Urbana, Illinois; and Division of Acute Care Surgery, Department of Surgery (E.R.H.), the Johns Hopkins University School of Medicine, Baltimore, Maryland.
Submitted: September 14, 2016, Accepted: September 17, 2016, Published online: October 25, 2016.
This study was presented at the 29th annual scientific assembly of the Eastern Association for Surgery of Trauma, January 12–16, 2016, in San Antonio, Texas.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s website (www.jtrauma.com).
Address for reprints: Vanessa P. Ho, MD, MPH, Division of Trauma, Surgical Critical Care, and Acute Care Surgery Case, Department of Surgery, Western Reserve University, 11100 Euclid Avenue, Cleveland, OH; email: Vanessa.Ho@uhhospitals.org.
Overview
Traumatic injuries to the pancreas are infrequent but can be associated with major morbidity and mortality, including acute hemorrhage, pancreatic leaks, abscesses, fistulae, and pancreatitis.[1] Estimates for the incidence of pancreatic injury range from 0.2% to 12% of abdominal traumas.[2–6] Many factors, such as patient stability, the acuity of concomitant life-threatening injuries, and the need for damage control procedures, must therefore be balanced when considering the proper approach to pancreatic injury management.
Historically, injuries to the pancreas were described by injury location as involving the head, body, and/or tail of the pancreas.[7–9] Early taxonomy for pancreatic injury did not require determination of involvement of the pancreatic duct, even though surgeons have long believed that ductal injury is the principal cause of pancreatic-specific morbidity and mortality.[10][11] The American Association for the Surgery of Trauma grading system, published in 1990, is a practical and prognostic way to describe pancreatic injury. With this system, typically, higher-grade injuries correlate with higher mortality and complications.[2][12] Grades I and II include minor pancreatic contusions and lacerations that spare the pancreatic duct. Grade III injuries include pancreatic duct injuries at the body and tail, and grade IV injuries include ductal injuries at the pancreatic head. Grade V injuries include massive disruption of the pancreatic head.
Computed tomography (CT) scan is the diagnostic modality of choice in hemodynamically stable blunt abdominal trauma patients to diagnose pancreatic injury. The sensitivities for detecting pancreatic injury are highly variable ranging from 47% to 79%, with newer-generation scanners being more sensitive.[13][14] Identification of pancreatic duct injury using CT imaging also varied, with sensitivities ranging from 52% to 54% with specificities between 90% and 95%.[13] Others have reported sensitivities from 91% to 95% with specificities of 91% to 100% pancreatic duct injury using multidetector CT scans.[15][16] Use of magnetic resonance cholangiopancreatography (MRCP) and endoscopic retrograde cholangiopancreatography (ERCP) for diagnostic tools for pancreatic injury are limited to case reports. However, the use of magnetic resonance imaging is believed to increase the diagnostic confidence of pancreatic injury according to Panda et al.[16] A review of the case series also showed that MRCP can be a useful tool for diagnostic purposes, whereas ERCP may provide diagnostic as well as therapeutic intervention but is limited due to the logistics of performing ERCP in general and the technical challenges of performing it in a multiple trauma patient with the risk of exacerbating the issue with pancreatitis.[17]
Therapeutic operative interventions for pancreatic injury are typically treated by drainage or suture repair for minor injuries, whereas more extensive injuries generally require pancreatic resection.[4] Surgeons have advocated various reconstruction options after resection, including gastrojejunostomy, Roux-en-Y reconstructions, and pancreaticoduodenectomy.[18] Commonly reported complications have included fistulae, pseudocysts, intraabdominal abscesses, and pancreatitis.[9] Pancreaticoduodenectomy was recommended by Foley and Fry[18] in 1969 as an aggressive approach for destructive pancreatic head injuries to curtail bleeding and ensure removal of all devitalized tissue. More recent advancements in surgical trauma care have introduced additional strategies, such as increased use of nonoperative management, endoscopic stenting for ductal injuries, and damage control surgery.[19–21] It is currently unknown which management strategies lead to the most favorable outcomes.
Our group investigated treatment strategies by severity (American Association for the Surgery of Trauma grade) of pancreatic injury. Additionally, we investigated two other common management decisions: first, whether octreotide should routinely be used after pancreatic surgery to prevent the development of pancreatic fistulae; and second, whether splenectomy should routinely be performed concomitant with distal pancreatectomy. To address these concerns in an objective and transparent manner, the Guidelines Section of the Eastern Association for the Surgery of Trauma used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology for this work.[22][23] The overall objective of this article was to provide evidence-based recommendations for the physician who is presented with traumatic injury to the pancreas.
Objectives
The objective of this guideline was to determine optimal treatment for patients with pancreatic injuries. We created a set of Population, Intervention, Control, Outcome (PICO) questions, as follows:
PICO 1
For adults with grade I/II injury to the pancreas identified by CT scan (P), should operative intervention (I) or nonoperative management (C) be performed?
PICO 2
For adults with grade III/IV injury to the pancreas identified by CT scan (P), should operative intervention (I) or nonoperative management (C) be performed?
PICO 3
For adults undergoing an operation who are intra-operatively found to have a grade I/II pancreas injury (P), should resectional (I) or nonresectional management (C) be performed?
PICO 4
For adults undergoing an operation who are intra-operatively found to have a grade III/IV pancreas injury (P), should resectional (I) or nonresectional management (C) be performed?
PICO 5
For adults with total destruction of the head of the pancreas (grade V) (P), should pancreaticoduodenectomy (I) or surgical treatment other than pancreaticoduodenectomy (C) be performed?
PICO 6
For adults who have undergone an operation for pancreatic trauma (P), should routine octreotide prophylaxis (I) or no octreotide (C) be used?
PICO 7
For adults undergoing distal pancreatectomy for trauma (P), should routine splenectomy (I) or splenic preservation (C) be performed?
Methodology
Outcome Measure Types
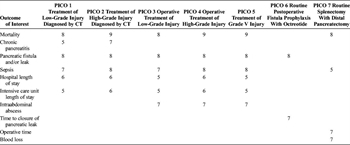
Table 1. Outcome Importance for Each PICO.
Relevant outcomes were established by the committee members a priori. Importance of each outcome was independently rated by each member of the subcommittee on a scale of 1 to 9 as described by the GRADE methodology.[23] Critical outcomes are scored 7 to 9, important outcomes are scored 4 to 6, and limited importance outcomes are scored 1 to 3. Outcome scores for each outcome for each PICO are presented in Table 1. Critical and important outcomes were considered in our review.
Identification of References
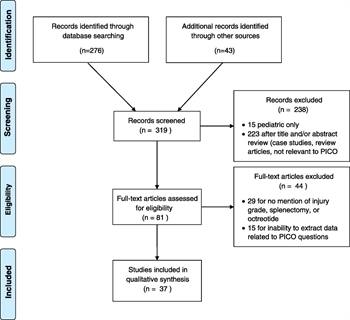
Figure 1. Included studies and PICO questions addressed.
A systematic search of the MEDLINE database using was performed on December 9, 2014, with the assistance of a professional librarian using the following search terms: (“Pancreas/surgery” [MeSH] AND (“wounds and injuries” [MeSH Terms] OR (“wounds” [All Fields] AND “injuries” [All Fields]) OR “wounds and injuries” [All Fields])). Related articles and bibliographies of included studies and reviews were searched manually. We only included English-language retrospective and prospective studies from January 1965 until December 2014. Articles that did not describe ductal injuries (either by anatomic description or by formal grading system) were excluded.
Three hundred nineteen articles were screened for relevance. Fifty-two articles were reviewed in full by the subcommittee members. Fifteen additional articles were excluded because data were not grouped by pancreatic injury severity or treatment methodology and outcomes could not be extracted. Thirty-seven articles were included for data extraction (Fig. 1); included articles were single or multiple institution retrospective studies or case series, as well as a single prospective randomized trial that compared closed suction and sump for postoperative drainage of the pancreas. Twenty-nine articles were reviewed for PICOs 1 to 5, two articles were reviewed for PICO 6, and 13 articles were reviewed for PICO 7.
Data Extraction and Methodology
Each article was reviewed by two subcommittee members to ensure concordance. If discordance occurred, a third subcommittee member re-reviewed the article. Data were then entered into a Microsoft Excel (Microsoft, Redmond, WA) spreadsheet. All entered data were checked in triplicate by the primary investigator to ensure accuracy. The quality of evidence was evaluated for each of the following domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias.
Within the literature, there was no uniform definition for pancreatic leak, fistula, sepsis, or mortality. Resectional management was defined as a procedure in which pancreatic tissue was removed by the surgeon in a manner that required transection of the pancreas (such as a distal pancreatectomy or a pancreaticoduodenectomy). Conversely, if no resection was performed, this was defined as nonresectional management; this generally included pancreatic repair, debridement, and placement of drains. Deaths were included if they were “pancreas-related” or not specified. Deaths attributed to causes other than the pancreatic injury were not extracted for pooled analysis but were noted for discussion. Intraoperative deaths and preoperative deaths were also not included in pooled analysis, because the committee felt that pancreatic injuries do not generally lead to immediate death; intraoperative and preoperative deaths are likely secondary to associated injuries. Pseudocysts and peripancreatic fluid collections that required intervention were included as pancreatic fistulae/leaks. Failure of nonoperative management was noted, although not a formal outcome for PICO questions, as a possible outcome for nonoperatively managed patients. This was defined as patients who required operative intervention after initial plan for nonoperative management. Data for each outcome were analyzed using STATA/SE, 14.0 (College Station, TX). Summary of findings tables were created using GRADEpro software (http://gdt.guidelinedevelopment.org/). Data were pooled and relative risk and risk differences were calculated, with 95% confidence intervals. Subcommittee members weighed the pooled data outcomes and literature quality to determine recommendations for each PICO question. The strength of the recommendations was based on the evidence, risk-versus-benefit ratio, and patient values.
Results
Results PICO 1
Treatment of Low-Grade Injury Diagnosed by Ct (PICO 1)
For adult patients with grade I/II injuries to the pancreas identified by CT scan, should operative intervention or nonoperative management be performed?
Qualitative Synthesis

Table 2. Treatment of Low-Grade Injury Diagnosed by CT (PICO 1).
Overall, 124 patients in 11 studies were identified (Table 2).[2][5][15][24–31] The quality of evidence was very low for all outcomes due to inadequate power, lack of direct comparisons between groups, varying definitions of outcomes, and limited reporting of outcomes. Of these, 62 patients in 3 studies were in the operative group, and 62 patients over 10 articles had no operation. There were no mortalities and no reports of sepsis in either group; there were also no reports of pancreatitis or fistula in the operatively managed group. Two (9.1%) of 22 nonoperativly managed patients developed pancreatitis and three (6.8%) of 44 nonoperatively managed patients developed a fistula. One nonoperatively managed patient with a pseudocyst died from an unrelated complication.[27] Length of stay (LOS) was not consistently reported; one article reported mean LOS to be 33 days in the operative group. Four other articles reported mean LOS to be from 10 to 24 days. Intensive care unit (ICU) LOS was reported in one article in each group and was 16 days. LOS data could not be pooled for statistical analysis.
The largest study to describe nonoperatively managed patients was by Lee et al,[28] which described outcomes of hemodynamically stable blunt trauma patients. All patients underwent contrast-enhanced CT scan with a 72-second delay to obtain portal-venous phase images. Lacerations of more than 50% of pancreatic thickness were classified as “highly likely” to have injury of the main pancreatic duct, which was verified by ERCP, MRCP, or surgery. Of 22 nonoperatively managed patients without duct injury, one developed a fistula.
The largest study to address the operative arm was by Teh et al.[15] This study described the ability of CT scan to diagnose pancreatic injury. Thirty-eight patients had a CT scan performed, of whom 22 had operative management, after imaging, for grade I/II injuries. There were no pancreas-related complications in this group. In this study, CT was 91% sensitive and 91% specific for the identification of pancreatic duct injury. Velmahos et al.[14] also reported CT diagnostic accuracy in a multicenter analysis of 230 blunt trauma patients. CT scan was performed for 200 of the 230 patients, and an injury was missed in 30 (15%), resulting in an overall sensitivity of 85%. This group reported no deaths attributable to low-grade pancreatic injuries, although outcomes were not stratified by treatment and were not included in pooled data.
Recommendation
We conditionally recommend nonoperative management for grade I/II pancreatic injuries diagnosed by CT scan. Nonoperative management appears to have low morbidity. If the pancreatic duct is not definitively intact, it seems reasonable to further evaluate the duct with additional tests, such as ERCP or MRCP, because this may change the grade of the injury and therefore the recommended treatment plan.
Results PICO 2
Treatment of High-Grade Injury Diagnosed by Ct (PICO 2)
For adult patients with grade III/IV injuries to the pancreas identified on CT scan, should operative intervention or nonoperative management be performed?
Qualitative Synthesis
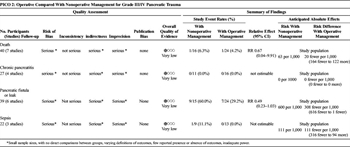
Table 3. Treatment of High-Grade Injury Diagnosed by CT (PICO 2).
Overall, 103 patients were identified in 8 articles (Table 3).[2][15][20][21][27][30–32] The quality of evidence was very low for all outcomes due to inadequate power, lack of direct comparisons between groups, varying definitions of outcomes, and limited reporting of outcomes of interest. Eighty-seven patients were operatively managed, and 16 patients were managed nonoperatively. Mortality data were available for 24 patients in the operative group and 16 in the nonoperative group; one patient died in each group. Twenty-nine percent (7 of 24) of operatively managed patients developed a fistula, compared with 60% (9 of 15) of patients who did not undergo an operation (p = 0.09). Sepsis was rarely reported, and there were no cases of chronic pancreatitis reported. For articles who reported patients who were operatively managed, mean LOS ranged from 17 to 104 days; this was 14 to 27 days in nonoperatively managed patients. These results could not be pooled to determine statistical significance.
The largest studies addressing this PICO were by Teh et al[15] (11 patients, all operatively managed), Kim et al,[27]and Pata et al.[30] (six patients, all nonoperatively managed). Kim et al.[27] described 11 patients; eight of those patients underwent an operation, whereas three patients underwent nonoperative management with stents placed at ERCP. All three nonoperatively managed patients had an intracapsular leak from the main pancreatic duct; two developed a pseudocyst. Of the eight patients who were managed with an operation, there were three pseudocysts; one patient died on hospital day 48 after developing an enterocutaneous fistula and respiratory failure. We included this death in our pooled analysis, because it is unclear if the pancreatic injury contributed to the patient's death. In the article by Teh et al,[15] 11 patients with ductal injury underwent distal pancreatic resection; none of these patients died from pancreas-related complications and one patient developed a low-output fistula that resolved after 5 weeks. Pata et al.[30] reported six nonoperatively managed patients with grade III injuries, of whom two developed fistulae. One death was noted in the nonoperative management group, which occurred in a blunt trauma patient who clinically declined after a pancreatic stent was placed at 28 hours, and subsequently had a distal pancreatectomy at 60 hours. This patient died from sepsis on hospital day 5.[20]
Nonoperative management failures are important for clinician consideration, but were not statistically analyzed because this outcome only pertains to the nonoperative group. The largest series describing failure of nonoperative management of pancreatic injuries was described by Velmahos et al.,[14] who reported 97 patients with blunt pancreatic and duodenal injuries (mostly grade I or grade II) who were initially managed nonoperatively. Nonoperative management failed in 10% (six pancreatic injuries, three duodenal injuries, and one with both). Four patients with grade III or IV injuries to the pancreas and duodenum had nonoperative management attempted, and two failed (50%), both requiring operations for clinical decline. Complications after failure of nonoperative management was 30%, compared with patients who did not require surgery (8%), although this was not statistically significant.
Recommendation
We conditionally recommend operative management for grade III/IV pancreatic injuries diagnosed by CT scan. Although there was no statistically significant difference between groups for any single outcome, our group feels that there is a cumulative trend toward increased morbidity after nonoperative management. Treatment failures after nonoperative management occur regularly, and treatment delays likely contribute to morbid complications and death.
Results PICO 3
Operative Management of Low-Grade Injury (PICO 3)
For adults undergoing an operation who are intraoperatively found to have a grade I/II pancreas injury, should resectional or nonresectional management be performed?
Qualitative Synthesis
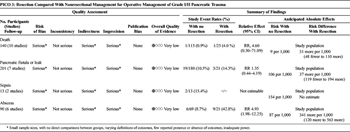
Table 4. Operative Management of Low-Grade Injury (PICO 3).
Overall, 299 patients were identified in 14 articles (Table 4).[5][24][25][29][31][33–41] The quality of evidence was very low for all outcomes due to inadequate power, lack of direct comparisons between groups, varying definitions of outcomes, and limited reporting of outcomes of interest. Twenty-seven patients were managed in the resection group, and 272 patients were managed in the nonresectional treatment group. Reported pancreas-related mortality in the resection group was 4.0% (1 of 25) versus 0.9% (1 of 115) in the nonresection group (p = 0.33). Fistula rates were 14.3% (3 of 21) in the resection group and 10.6% (19 of 180) the nonresection group (p = 0.7). Sepsis was not reported in the resection group, but developed in 2 (15.4%) of 13 nonresection patients. Intra-abdominal abscess formation was significantly higher in the resection group (42.9%) than in the nonresection group (8.7%) (p = 0.0009). LOS was not reported in the resection group; for patients without resection, mean LOS ranged from 7 to 27 days, and mean ICU LOS was reported in one article as 9 days. Again, LOS data could not be pooled for statistical analysis.
Many patients with a grade I or II pancreatic injury underwent operations to treat injuries to other organs. Pancreatic injury was often an incidental finding and was not surgically treated, or treated with drainage alone. Generally, patients had low complication rates and low mortality. Few articles reported resection for grade II injuries, although it may be difficult to distinguish a grade II or III injury in the operating room if the duct is not clearly visualized.
The largest contributor of data for the resection group was the article by Cogbill et al.[34] This group studied 74 patients who had pancreatic injuries managed by distal pancreatectomy, including 19 patients with grade II injuries. There was a single mortality (5%) within this group, eight (42%) intra-abdominal abscesses, and three (16%) pancreatic fistulae. It was not noted whether this death was attributable to pancreas-related morbidity, and it was included in the pooled analysis. Nonresection treatment strategies included pancreatography, drainage alone, or no drainage. There was one death, after a grade II injury treated by pancreatography, who developed pancreatic necrosis, sepsis, and multiple organ failure.[5]
Recommendation
We conditionally recommend nonresectional management for operative management of grade I/II pancreatic injuries. Our pooled data analysis suggests that mortality from pancreas-related causes are generally low in this population and that there were significantly more intra-abdominal abscesses in the resection group.
Results PICO 4
Operative Management of High-Grade Injury (PICO 4)
For adults already undergoing an operation who are intraoperatively found to have a grade III/IV pancreas injury, should resection or nonresection be performed?
Qualitative Synthesis
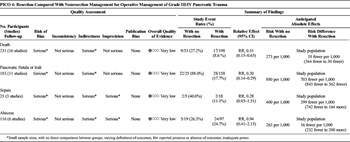
Table 5. Operative Management of High-Grade Injury (PICO 4).
Overall, 314 patients were identified in 19 articles (Table 5).[15][19–21][24][25][29][31–42] The overall quality of evidence was very low for all outcomes due to inadequate power, lack of direct comparisons between groups, varying definitions of outcomes, and limited reporting of outcomes of interest. Of these, 275 patients were managed in the resection group and 39 patients were managed in the nonresection group. Mortality was significantly lower in the resection group than the nonresection group (8.6% vs. 27.2%, p = 0.005), as were fistulae (17.7% vs. 88.0%, p < 0.0001). Sepsis was infrequently reported, but was 11.1% (2 of 18) in the resection group and 40% (2 of 5) in non-resection group (p = 0.19). Intra-abdominal abscess formation was reported in 24.7% (24 of 97) patients in the resection group and 26.3% (5 of 19) patients in the non-resection group, p = 1. Mean hospital LOS range for the resection group was 21 to 22 days and 24 to 42 days in the nonresection group. Mean ICU LOS was reported in one study with six resected patients and was 6 days. LOS was not uniformly reported in a way that could be pooled for statistical analysis.
Mortality was difficult to extract. Of note, many studies were published before the widespread use of damage control principles. Many patients who did not receive a resection had concomitant injuries precluding intervention, which may be a confounder for higher mortality. Additionally, there were a higher number of ambiguous mortalities (unspecified whether they were pancreas-related) in the nonresectional group, whereas deaths in the resection group were specifically reported to be unrelated to the pancreatic injury and were excluded. If all-cause mortality is counted for both groups, mortality for the resection group rises, and the difference between groups is no longer statistically significant (15% vs. 27.2%, p = 0.07).
Patients with no resection typically had drainage with or without repair of pancreatic parenchyma. Two articles focusing on outcomes after “conservative” (no resection) management reported that all patients without resections developed fistulae.[29][36] In the resection group, fistula formation ranged from 10% to 50%.[15][20][24][25][31][34][39–41]These articles described that delayed pancreatic injury diagnosis was accompanied by a high complication rate; additionally, multiple articles described patients with missed injuries at the initial evaluation who subsequently died from sepsis.[32][37][38][40] High-grade pancreatic injuries should be promptly evaluated to ensure expeditious treatment.
Recommendation
We conditionally recommend resection for operative management of grade III/IV pancreatic injuries. Complications are frequent in both groups. In our pooled analysis, fistula development was associated with nonresection strategies. Pancreas-related mortality was higher in the nonresection group, but this finding was potentially confounded by incomplete mortality reporting and bias. Due to the very low quality of available data, this is a conditional recommendation.
Results PICO 5
Treatment of Grade V Injury (PICO 5)
For adults with total destruction of the head of the pancreas (grade V), should pancreaticoduodenectomy or surgical treatment other than pancreaticoduodenectomy be performed?
Qualitative Synthesis
Forty-one patients were identified in 13 articles, (see Tables, Supplemental Digital Content 1, http://links.lww.com/TA/A830).[19][24][25][31][32][36][38][40][41][43–45] The quality of evidence was very low for all outcomes due to very small groups and inadequate power, lack of direct comparisons between groups, varying definitions of outcomes, and limited reporting of outcomes of interest. Of these, 38 patients had a pancreaticoduodenectomy and five patients were managed without pancreaticoduodenectomy. Reported postoperative mortality was 33.3% (12 of 36) after pancreaticoduodenectomy and 40% (2 of 5) in the non-pancreaticoduodenectomy group (p = 1). Fistula rates, sepsis, and intraabdominal abscess formation were not significantly different between groups (all p > 0.05), likely due to very small sample sizes. Mean hospital LOS was reported in one study of three pancreaticoduodenectomy patients (24 days); LOS was reported as 28 days for one non-pancreaticoduodenectomy patient, with 7 days in ICU.
As described in Patients and Methods, intraoperative and preoperative deaths are not included in our pooled analysis above. When these deaths are considered, mortality for these injuries rises to 73%. Because some studies did not report preoperative deaths, the true mortality from injuries associated with grade V injuries may exceed 73%. Four different surgeries were attempted on the five patients in the non-pancreaticoduodenectomy group. Two underwent damage control, both of whom died before a definitive procedure could be attempted.[19] One patient survived after debridement and packing and developed a fistula.[24] Two patients survived: one with a pyloric exclusion, gastroenterostomy, and drainage,[43] and the other after a pancreaticojejunostomy with a Roux-en-Y reconstruction.[40] Three studies reported the use of damage control methods. Amongst these studies, mortality was also high, at 27.3% (3 of 11 patients), with all three of the deaths occurring between the initial damage control procedure and definitive management.
Recommendation
No recommendation is given. The literature on this topic is limited and dated. Surgical and resuscitation strategies have evolved significantly to include damage control procedures and early balanced resuscitations, making our ability to interpret the available literature limited. Grade V injury to the pancreas is extremely morbid, and the intraoperative and immediate postoperative rate of death is high.
Results PICO 6
Routine Postoperative Fistula Prophylaxis With Octreotide (PICO 6)
For adult patients who have undergone an operation for pancreatic trauma, should routine octreotide prophylaxis or no octreotide be used?
Qualitative Synthesis
Somatostatin analogues have been used in elective surgery for reduction of clinically significant pancreatic leak/fistula. The use of octreotide to reduce pancreatic leak have had mixed results. Multiple studies in Europe have found to have reduced rates of pancreatic leak or fistula; however, similar studies in the United States as well as meta-analysis have not concurred.[46–48] Thus, routine use of octreotide has not been advocated for pancreatic leak or prevention of leak in elective surgery. Allen et al.[49] showed that the use of Pasireotide, a somatostatin analogue that has a longer half-life than octreotide reduced the postoperative leak, fistula, abscess rate when compared with placebo (9% vs. 21%, p = 0.006). This analogue has not been studied in the trauma patient population, and it is unclear whether these results would translate to the pancreatic leak/fistula rates in blunt pancreatic injury patients.

Table 6. Routine Postoperative Fistula Prophylaxis With Octeotide (PICO 6).
Two studies addressed the routine use of octreotide after pancreatic injury (Table 6).[50][51] The quality of evidence was very low, with no direct comparisons between groups, imprecise outcomes definitions, few reported outcomes, inadequate power, and dissimilar estimates of outcomes between studies. No difference was found for the development of fistulae between patients who received octreotide in the postoperative setting (35.7%) and those who did not (36.8%, p = 0.8). Additional uses for octreotide in the literature included fistula treatment and use as an adjunct to nonoperative management; these were not included in our analysis.
Nwariaku et al.[51] retrospectively studied 90 patients diagnosed intraoperatively with pancreatic injury. Of 80 survivors, 21 patients received octreotide (100 μg every 8 hours) and 55 did not; administration was not protocolized. The group that received octreotide had more severe pancreatic injuries (grades III, IV, V) compared with the group that did not (38% vs. 16%), but this was not statistically significant. Patients underwent a variety of procedures, including drainage, resection, and pancreaticoduodenectomy. The overall fistula rate was 40%, and there was no significant difference in fistula rate between patients who did and did not receive octreotide. In the subgroup of patients with higher-grade injuries (grades III-V), the fistula rate was 53%, with no difference between groups. There was also no statistical difference in duration of fistula drainage (25 ± 5 days in the octreotide group vs. 16 ± 2 days in the no octreotide group).
Amirata et al.[50] described 28 patients with pancreatic injury, of whom seven were treated with octreotide. Dosing was inconsistent, ranging from 150 to 600 μg per day. All seven patients treated with postoperative octreotide developed no complications, whereas six of the 21 patients not treated with octreotide developed pancreatic complications.
Recommendation
We conditionally recommend against the routine use of octreotide for postoperative prophylaxis related to traumatic pancreatic injuries to prevent fistula. Data are limited, but pooled data show no difference in outcomes between groups. The subcommittee concluded that the less invasive (no medication) strategy would be preferable with no difference in outcomes.
Results PICO 7
Routine Splenectomy With Distal Pancreatectomy (PICO 7)
For adults undergoing a distal pancreatectomy for trauma, should routine splenectomy or splenic preservation be performed?
Qualitative Synthesis
Two hundred thirty-four patients were identified in 13 articles, (see Tables, Supplemental Digital Content 1, http://links.lww.com/TA/A830).[28][32][35][37][38][40][42][52–56] The quality of evidence was very low due to lack of direct comparisons between groups, varying definitions of outcomes, few reported outcomes, and inadequate power. Splenectomy was performed in 154 patients, and 80 patients had a splenic preserving distal pancreatectomy. Splenic preservation was only used for hemodynamically stable patients. Mortality was similar, 9.2% in the splenectomy group and 7.7% after splenic preservation (p = 0.49). Postoperative sepsis was also similar (21.1% vs. 21.6%, p = 1). Overwhelming postsplenectomy infection (OPSI) and blood loss were not reported. Total operative time was reported in two articles; mean operative time for distal pancreatectomy with splenectomy was 164 minutes in one article versus 285 minutes for a spleen-preserving distal pancreatectomy in a different article; these raw numbers do not account for additional procedures. Pachter et al[53] found that the operative time for the pancreatectomy part of the operation for patients who had splenic preservation was 51 minutes.
Splenic preservation can be technically challenging and is more time-consuming than a distal pancreatectomy but leads to the future benefit of a decreased risk of OPSI. Estimates suggest that the lifetime risk of OPSI is approximately 5% for patients who receive splenectomy for hematologic disorders, and lower for trauma patients.[57] One study suggests that the incidence of severe late OPSI after trauma splenectomy was 0.21 per 1000 person-years of exposure, with the majority occurring greater than 5 years after splenectomy.[58] No reports of OPSI were found in our review.
In these articles, mortality causes were ambiguous, but there was no difference in mortality between groups. Of note, one study of six cases of splenic salvage[55] reported two severe complications related to bleeding, one patient died from hemorrhagic shock, which developed within 12 hours postoperatively. One additional patient had a return to the OR for bleeding from the splenic vein and required a delayed splenectomy.
Recommendations
No recommendation is given. Existing data do not support either treatment modality, although splenic preservation was only considered for stable patients. If either the stability of the patient or the surgeon’s ability to safely preserve the spleen is in doubt, a distal pancreatectomy with splenectomy is a reasonable choice.
Using These Guidelines in Clinical Practice
These guidelines represent a detailed summary of the literature regarding treatment for pancreatic trauma. Most studies are from large trauma centers and may not be applicable to all centers or all situations and are intended to inform the decision-making process rather than to replace clinical judgment. Pancreatic injuries without involvement of the pancreatic duct appear to have low morbidity, and therefore management without resection appears to be safe. Higher-grade injuries involving the pancreatic duct have increased attributable morbidity and mortality as well as potential for deterioration if treatment is delayed, and literature supports resection in these cases.
Conclusion
In summary, we propose the following recommendations:
(1) For adult patients with grade I or II injury to the pancreas identified on CT scan, we conditionally recommend nonoperative management.
(2) For adult patients with grade III or IV injury to the pancreas identified on CT scan, we conditionally recommend operative intervention.
(3) For adult patients with grade I or II injuries to the pancreas who are undergoing an operation, we conditionally recommend non-resectional management.
(4) For adult patients with grade III or IV injuries to the pancreas who are undergoing an operation, we conditionally recommend resectional management.
(5) For adult patients with grade V injuries to the pancreas who are undergoing an operation, we give no recommendation regarding whether a pancreaticoduodenectomy or a surgical procedure other than pancreaticoduodenectomy should be performed.
(6) For adult patients who have undergone an operation for pancreatic trauma, we conditionally recommend against the routine use of octreotide prophylaxis.
(7) For adult patients undergoing a distal pancreatectomy for pancreatic trauma, we give no recommendation regarding whether routine splenectomy or splenic preservation should be performed.
Disclosure
Dr. Bokhari was a Bristol Myers Squibb panel participant in last 36 months and is on the Speaker panel for Abbott Point of Care.
Dr. Haut is the primary investigator of a contract (CE-12-11-4489) with the Patient-Centered Outcomes Research Institute (PCORI), entitled “Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient-Centered Care via Health Information Technology.” Dr. Haut receives royalties from Lippincott, Williams, Wilkins for a book, Avoiding Common ICU Errors. Dr. Haut is a paid consultant and speaker for the “Preventing Avoidable Venous Thromboembolism—Every Patient, Every Time” VHA IMPERATIV® Advantage Performance Improvement Collaborative and the Illinois Surgical Quality Improvement Collaborative.
Source of Funding: None.
References
- Akhrass R, Yaffe MB, Brandt CP, Reigle M, Fallon WF Jr, Malangoni MA. Pancreatic trauma: a ten-year multi-institutional experience. Am Surg. 1997;63(7):598–604.
- Heuer M, Hussmann B, Lefering R, Taeger G, Kaiser GM, Paul A, Lendemans S. Pancreatic injury in 284 patients with severe abdominal trauma: outcome, course, and treatment algorithm. Langenbecks Arch Surg. 2011;396(7):1067–1076.
- Antonacci N, Di Saverio S, Ciaroni V, Biscardi A, Giugni A, Cancellieri F, Coniglio C, Cavallo P, Giorgini E, Baldoni F, et al. Prognosis and treatment of pancreaticoduodenal traumatic injuries: which factors are predictors of outcome? J Hepatobiliary Pancreat Sci. 2011;18(2):195–201.
- Jurkovich GJ, Carrico CJ. Pancreatic trauma. Surg Clin North Am. 1990;70(3):575–593.
- Timberlake GA. Blunt pancreatic trauma: experience at a rural referral center. Am Surg. 1997;63(3):282–286.
- Scollay JM, Yip VS, Garden OJ, Parks RW. A population-based study of pancreatic trauma in Scotland. World J Surg. 2006;30(12):2136–2141.
- Sheldon GF, Cohn LH, Blaisdell FW. Surgical treatment of pancreatic trauma. J Trauma. 1970;10(9):795–800.
- Sims EH, Mandal AK, Schlater T, Fleming AW, Lou MA. Factors affecting outcome in pancreatic trauma. J Trauma. 1984;24(2):125–128.
- Smith AD Jr, Woolverton WC, Weichert RF 3rd, Drapanas T. Operative management of pancreatic and duodenal injuries. J Trauma. 1971;11(7):570–576.
- Lucas CE. Diagnosis and treatment of pancreatic and duodenal injury. Surg Clin North Am. 1977;57(1):49–65.
- Bradley EL 3rd. Chronic obstructive pancreatitis as a delayed complication of pancreatic trauma. HPB Surg. 1991;5(1):49–59; discussion −60.
- Kao LS, Bulger EM, Parks DL, Byrd GF, Jurkovich GJ. Predictors of morbidity after traumatic pancreatic injury. J Trauma. 2003;55(5):898–905.
- Phelan HA, Velmahos GC, Jurkovich GJ, Friese RS, Minei JP, Menaker JA, Philp A, Evans HL, Gunn ML, Eastman AL, et al. An evaluation of multidetector computed tomography in detecting pancreatic injury: results of a multicenter AAST study. J Trauma. 2009;66(3):641–646; discussion 6–7.
- Velmahos GC, Tabbara M, Gross R, Willette P, Hirsch E, Burke P, Emhoff T, Gupta R, Winchell RJ, Patterson LA, et al. Blunt pancreatoduodenal injury: a multicenter study of the Research Consortium of New England Centers for Trauma (ReCONECT). Arch Surg. 2009;144(5):413–419; discussion 9–20.
- Teh SH, Sheppard BC, Mullins RJ, Schreiber MA, Mayberry JC. Diagnosis and management of blunt pancreatic ductal injury in the era of high-resolution computed axial tomography. Am J Surg. 2007;193(5):641–643; discussion 3.
- Panda A, Kumar A, Gamanagatti S, Bhalla AS, Sharma R, Kumar S, Mishra B. Evaluation of diagnostic utility of multidetector computed tomography and magnetic resonance imaging in blunt pancreatic trauma: a prospective study. Acta Radiol. 2015;56(4):387–396.
- Bhasin DK, Rana SS, Rawal P. Endoscopic retrograde pancreatography in pancreatic trauma: need to break the mental barrier. J Gastroenterol Hepatol. 2009;24(5):720–728.
- Foley WJ, Fry WJ. Pancreatic trauma. Postgrad Med. 1969;45(6):106–109.
- Lee KJ, Kwon J, Kim J, Jung K. Management of blunt pancreatic injury by applying the principles of damage control surgery: experience at a single institution. Hepatogastroenterology. 2012;59(118):1970–1975.
- Lin BC, Liu NJ, Fang JF, Kao YC. Long-term results of endoscopic stent in the management of blunt major pancreatic duct injury. Surg Endosc. 2006;20(10):1551–1555.
- Hamidian Jahromi A, D'Agostino HR, Zibari GB, Chu QD, Clark C, Shokouh-Amiri H. Surgical versus nonsurgical management of traumatic major pancreatic duct transection: institutional experience and review of the literature. Pancreas. 2013;42(1):76–87.
- Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, Dahm P. Eastern Association for the Surgery of Trauma Practice Management Guidelines Ad Hoc C. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. The Journal of Trauma and Acute Care Surgery. 2012;73(5 Suppl 4):S283–S287.
- Callcut RA, Como J, Patel MB, Velopulos C, Chiu WC, Kerwin AJ, Lau BD, Ferrada P, Dahm P, Sultan S, Falck-Ytter Y, Robinson BRH, Haut ER. Writing an EAST Practice Management Guideline (PMG): A Step-By-Step How-To-Guide. 2015. https://www.east.org/education/treatment-guidelines/using-grade-in-east-practice-management-guidelines. 2015.
- Al-Ahmadi K, Ahmed N. Outcomes after pancreatic trauma: experience at a single institution. Can J Surg. 2008;51(2):118–124.
- el-Boghdadly S, al-Yousef Z, al Bedah K. Pancreatic injury: an audit and a practical approach. Ann R Coll Surg Engl. 2000;82(4):258–262.
- Kaman L, Iqbal J, Pall M, Bhukal I, Behera A, Singh G, Singh R. Current management of pancreatic trauma. Trop Gastroenterol. 2012;33(3):200–206.
- Kim HS, Lee DK, Kim IW, Baik SK, Kwon SO, Park JW, Cho NC, Rhoe BS. The role of endoscopic retrograde pancreatography in the treatment of traumatic pancreatic duct injury. Gastrointest Endosc. 2001;54(1):49–55.
- Lee PH, Lee SK, Kim GU, Hong SK, Kim JH, Hyun YS, Park DH, Lee SS, Seo DW, Kim MH. Outcomes of hemodynamically stable patients with pancreatic injury after blunt abdominal trauma. Pancreatology. 2012;12(6):487–492.
- Lewis G, Knottenbelt JD, Krige JE. Conservative surgery for trauma to the pancreatic head: is it safe? Injury. 1991;22(5):372–374.
- Pata G, Casella C, Di Betta E, Grazioli L, Salerni B. Extension of nonoperative management of blunt pancreatic trauma to include grade III injuries: a safety analysis. World J Surg. 2009;33(8):1611–1617.
- Young PR Jr, Meredith JW, Baker CC, Thomason MH, Chang MC. Pancreatic injuries resulting from penetrating trauma: a multi-institution review. Am Surg. 1998;64(9):838–843; discussion 43–4.
- Lin BC, Chen RJ, Fang JF, Hsu YP, Kao YC, Kao JL. Management of blunt major pancreatic injury. J Trauma. 2004;56(4):774–778.
- Ahmad I, Branicki FJ, Ramadhan K, El-Ashaal Y, Abu-Zidan FM. Pancreatic injuries in the United Arab Emirates. Scand J Surg. 2008;97(3):243–247.
- Cogbill TH, Moore EE, Morris JA Jr, Hoyt DB, Jurkovich GJ, Mucha P Jr, Ross SE, Feliciano DV, Shackford SR. Distal pancreatectomy for trauma: a multicenter experience. J Trauma. 1991;31(12):1600–1606.
- Degiannis E, Levy RD, Potokar T, Lennox H, Rowse A, Saadia R. Distal pancreatectomy for gunshot injuries of the distal pancreas. Br J Surg. 1995;82(9):1240–1242.
- Degiannis E, Levy RD, Velmahos GC, Potokar T, Florizoone MG, Saadia R. Gunshot injuries of the head of the pancreas: conservative approach. World J Surg. 1996;20(1):68–71; discussion 2.
- Fabian TC, Kudsk KA, Croce MA, Payne LW, Mangiante EC, Voeller GR, Britt LG. Superiority of closed suction drainage for pancreatic trauma. A randomized, prospective study. Ann Surg. 1990;211(6):724–728; discussion 8–30.
- Olah A, Issekutz A, Haulik L, Makay R. Pancreatic transection from blunt abdominal trauma: early versus delayed diagnosis and surgical management. Dig Surg. 2003;20(5):408–414.
- Rickard MJ, Brohi K, Bautz PC. Pancreatic and duodenal injuries: keep it simple. ANZ J Surg. 2005;75(7):581–586.
- Sukul K, Lont HE, Johannes EJ. Management of pancreatic injuries. Hepatogastroenterology. 1992;39(5):447–450.
- Krige JE, Kotze UK, Hameed M, Nicol AJ, Navsaria PH. Pancreatic injuries after blunt abdominal trauma: an analysis of 110 patients treated at a level 1 trauma centre. S Afr J Surg. 2011;49(2: 58, 60, 2–4 passim).
- Robey E, Mullen JT, Schwab CW. Blunt transection of the pancrease treated by distal pancreatectomy, splenic salvage and hyperalimentation. Four cases and review of the literature. Ann Surg. 1982;196(6):695–699.
- Ivatury RR, Nallathambi M, Rao P, Stahl WM. Penetrating pancreatic injuries. Analysis of 103 consecutive cases. Am Surg. 1990;56(2):90–95.
- Nance FC, DeLoach DH. Pancreaticoduodenectomy following abdominal trauma. J Trauma. 1971;11(7):577–585.
- Salam A, Warren WD, Kalser M, Laguna V. Pancreatoduodenectomy for trauma: clinical and metabolic studies. Ann Surg. 1972;175(5):663–672.
- Lowy AM, Lee JE, Pisters PW, Davidson BS, Fenoglio CJ, Stanford P, Jinnah R, Evans DB. Prospective, randomized trial of octreotide to prevent pancreatic fistula after pancreaticoduodenectomy for malignant disease. Ann Surg. 1997;226(5):632–641.
- Yeo CJ, Cameron JL, Lillemoe KD, Sauter PK, Coleman J, Sohn TA, Campbell KA, Choti MA. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg. 2000;232(3):419–429.
- Jin K, Zhou H, Zhang J, Wang W, Sun Y, Ruan C, Hu Z, Wang Y. Systematic review and meta-analysis of somatostatin analogues in the prevention of postoperative complication after pancreaticoduodenectomy. Dig Surg. 2015;32(3):196–207.
- Allen PJ, Gönen M, Brennan MF, Bucknor AA, Robinson LM, Pappas MM, Carlucci KE, D'Angelica MI, DeMatteo RP, Kingham TP, et al. Pasireotide for postoperative pancreatic fistula. N Engl J Med. 2014;370(21):2014–2022.
- Amirata E, Livingston DH, Elcavage J. Octreotide acetate decreases pancreatic complications after pancreatic trauma. Am J Surg. 1994;168(4):345–347.
- Nwariaku FE, Terracina A, Mileski WJ, Minei JP, Carrico CJ. Is octreotide beneficial following pancreatic injury? Am J Surg. 1995;170(6):582–585.
- Ivatury RR, Simon RJ, Guignard J, Kazigo J, Gunduz Y, Stahl WM. The spleen at risk after penetrating trauma. J Trauma. 1993;35(3):409–414.
- Pachter HL, Hofstetter SR, Liang HG, Hoballah J. Traumatic injuries to the pancreas: the role of distal pancreatectomy with splenic preservation. J Trauma. 1989;29(10):1352–1355.
- Sriussadaporn S. Management of pancreatic injuries. J Med Assoc Thai. 1994;77(11):580–587.
- Yadav TD, Natarajan SK, Kishore VM, Lyngdoh S, Wig JD. Spleen-preserving distal pancreatectomy for pancreatic trauma: a series of six cases. JOP. 2007;8(4):422–428.
- Yalin K, Xiaojun H, Chengli L, Gang Z, Mei X, Yuying Z, Hongyi Z. Grading-therapeutic strategy for pancreatic injury after blunt abdominal trauma: therapy based on the condition of pancreatic duct and report of 95 cases. Hepatogastroenterology. 2013;60(126):1497–1503.
- Okabayashi T, Hanazaki K. Overwhelming postsplenectomy infection syndrome in adults - a clinically preventable disease. World J Gastroenterol. 2008;14(2):176–179.
- Cullingford GL, Watkins DN, Watts AD, Mallon DF. Severe late postsplenectomy infection. Br J Surg. 1991;78(6):716–721.
Supplemental Digital Content