Geriatric Trauma, Evaluation and Management of
Archived PMG
Published 2012
Citation: J Trauma. 73(5):S345-S350, November 2012
Authors
Calland, James Forrest MD; Ingraham, Angela M. MD; Martin, Niels MD; Marshall, Gary T. MD; Schulman, Carl I. MD, PhD, MSPH; Stapleton, Tristan; Barraco, Robert D. MD, MPH
Author Information
From the Department of Surgery (J.F.C.), University of Virginia, Charlottesville, Virginia; Department of Surgery (A.M.I.), University of Cincinnati, Cincinnati, Ohio; Jefferson University School of Medicine (N.M.), Philadelphia, Pennsylvania; University of Pittsburgh Medical Center (G.T.M.), Pittsburgh, Pennsylvania; DeWitt Daughtry Family Department of Surgery (C.I.S.), University of Miami Miller School of Medicine, Miami, Florida; Department of Surgery (T.S.), University of Virginia School of Medicine, Charlottesville, Virginia; and University of South Florida College of Medicine Lehigh Valley Health Network (R.D.B.), Allentown, Pennsylvania.
Address for reprints: James Forrest Calland, MD, FACS, Box 800709, Department of Surgery, University of Virginia, Charlottesville, VA 22908; email: calland@virginia.edu.
Introduction
Elderly trauma patients face an increased risk for adverse outcomes after injury. As such, clinicians treating injured patients of advanced age need guidance in identifying the techniques and practices that have the proven capacity to improve outcomes. Although independent risk for postinjury mortality may begin at a much younger age, the authors of this analysis have chosen to limit their recommendations to those patients aged 65 years or older. This threshold is consonant with what seems to be the most common assumptions and designations of existing trauma centers regarding advancing age.
Statement of the Problem
As it pertains to the care of the injured, “triage” is variously defined as “…the sorting of and allocation of treatment to patients and especially battle and disaster victims according to a system of priorities designed to maximize the number of survivors.”[1] For the elderly patient, it is often difficult to accurately identify the severity of injury and the degree of physiologic derangement because of age-related differences in biology.[2][3] In addition, there also exists a complex interplay of social and cultural determinants that likely account for why many elderly trauma patients are not approached with the same aggressive form of evaluation afforded to younger patients.
Among academic trauma surgeons, a substantial difference of opinion seems to exist on whether falls from standing or hip fractures qualify as a “geriatric trauma” worthy of admission to a dedicated trauma service. This ambivalence seems to extend into the community where there exists substantial evidence that elderly patients are less likely to be referred to trauma centers—perhaps because of conflicting experimental evidence on the survival of the elderly injured patient when treated at designated trauma centers.[4–12]
Clinical problems (in injured elderly patients) addressed by this guideline are as follows:
- Is advanced age a triage criterion for trauma center referral and activation?
- Is an elevated base deficit a surrogate for severe injury and the need for intensive care?
- Should withdrawal or limitation of care be initiated solely on the basis of advancing age?
- What is the influence of preexisting conditions and complications in injury-related outcomes?
- How should medication-induced coagulopathy be treated?
- Is it useful to attempt supraphysiologic resuscitation after injury?
The above items are the issues addressed by this update. One additional issue with particular relevance to the elderly not addressed by this update is the use of epidural catheters after blunt thoracic trauma because it is adequately covered elsewhere by a separate guideline.[13]
Process
An initial database query was undertaken using MEDLINE, with citations published between the years 2000 and 2008. Using the search words “geriatric,” “trauma,” “elderly,” and “injury” and by limiting the search to citations dealing with human subjects and published in the English language, more than 400 citations were identified. Letters to the editor, case reports, reviews, and articles dealing with minor injury mechanisms, particularly hip fractures from slip and falls, were then excluded. The abstracts of the remaining citations were each reviewed, and those articles that did not address the issues pertinent to the three aims of this review and patient age criteria, 65 years or older, were further excluded. This yielded a total of 64 articles that comprised the initial evidentiary table. The bibliographies of these 64 articles were then further reviewed, and eight additional articles meeting the previously mentioned criteria were added for a total of 90 references within the evidentiary table. Each reference was then reviewed by two trauma surgeons, and a consensus was reached regarding appropriate classification of each reference according to the Eastern Association for the Surgery of Trauma (EAST) primer on evidence-based medicine. Eighteen articles were subsequently excluded from the evidentiary table after being identified as pure review articles with no new synthesis of information.
Criteria for achieving a specific classification in the final evidentiary table and the number of articles for each class are shown below:
Class I: Prospective randomized controlled trials—the gold standard of clinical trials. Some may be poorly designed, have inadequate numbers, or have other methodological inadequacies (0 references).
Class II: Clinical studies in which data were collected prospectively and retrospective analyses that were based on clearly reliable data. Types of studies so classified include observational studies, cohort studies, prevalence studies, and case-control studies (38 references).
Class III: Studies based on retrospectively collected data. Evidence used in this class includes clinical series, database or registry reviews, large series of case reviews, and expert opinion (35 references).
The EAST primer on using evidence-based outcome measures to develop practice management guidelines (PMGs) suggested the levels of recommendation (Level 1, Level 2, Level 3, etc.) that were used to generate the following conclusions and summary recommendations.[14]
Recommendations
Question 1
Should age be an independent determinant of triage decisions such as whether trauma patients receive care as a trauma team “alert” at a designated trauma center or in decision making related to limiting care?
Level 1
- There are insufficient Class I and Class II data to support any standards regarding any of the questions posed by this query.
Level 2
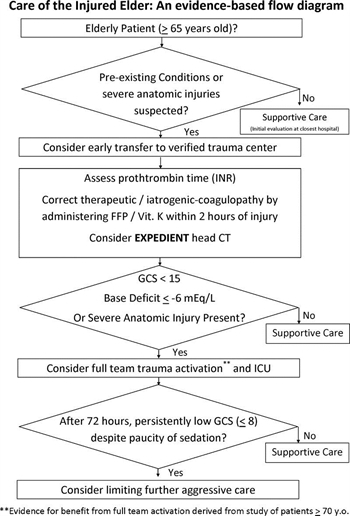
- Injured patients with advanced age (aged ≥65 years) and preexisting medical conditions should lower the threshold for field triage directly to a designated/verified trauma center.
- Advanced patient age is not an absolute predictor of poor outcomes following trauma and, therefore, should NOT be used as the sole criterion for denying or limiting care in this patient population.
- An initial aggressive approach should be pursued for management of the elderly patient unless in the judgment of an experienced trauma surgeon it seems that the injury burden is severe and the patient appears moribund.
Level 3
- A lower threshold for trauma activation should be used for injured patients aged 65 years or older who are evaluated at trauma centers.
- Elderly patients with severe anatomic injuries (e.g., one or more body systems with an Abbreviated Injury Scale [AIS] score of ≥3) should be treated in designated trauma centers, preferably in intensive care units (ICUs) staffed by surgeon-intensivists.
- In patients aged 65 years or older with a Glasgow Coma Scale (GCS) score less than 8, if substantial improvement in GCS is not realized within 72 hours of injury, consideration should be given to limiting further aggressive therapeutic interventions.
Question 2
How should medication-induced coagulopathy be addressed during the early postinjury period?
Level 1
- There are insufficient Class I and Class II data to support any standards regarding any of the questions posed by this query.
Level 2
- There are insufficient Class I and Class II data to support any standards regarding any of the questions posed by this query.
Level 3
- All elderly patients who were taking medications for systemic anticoagulation before their injury should have appropriate assessment of their coagulation profile as soon as possible after admission.
- All elderly patients with suspected head injury (e.g., those with altered GCS, headache, nausea, external trauma, or high-energy mechanism) who were taking medications for systemic anticoagulation before their injury should be evaluated with head computed tomography as soon as possible after admission.
- Patients receiving warfarin with a posttraumatic intracranial hemorrhage should receive initiation of therapy to correct their international normalized ratio (INR) toward a normal range (e.g., <1.6× normal) within 2 hours of admission.
Question 3
Is indiscriminate invasive cardiovascular monitoring with pulmonary artery catheters and supranormal resuscitation still justified after injury in older patients?
Level 1
- There are insufficient Class I and Class II data to support any standards regarding any of the questions posed by this query.
Level 2
- There are insufficient Class I and Class II data to support any standards regarding any of the questions posed by this query.
Level 3
- Elderly patients with one or more severe anatomic injuries (i.e., one or more body system AIS score of ≥3) should be treated in designated trauma centers, preferably in ICUs staffed by surgeon-intensivists.
- Base deficit measurements may provide useful information in determining the status of initial resuscitation and risk of mortality for geriatric patients. As such, ICU admission should be considered for patients aged 65 years or older with an initial base deficit of −6 mEq/L or less.
Scientific Foundation
Triage and Advanced Age
One of the main topics addressed by this PMG is the manner in which elderly patients are triaged to trauma centers and, if triaged to a trauma center, whether they should routinely receive a trauma activation level of initial care and what is an appropriate threshold for admitting them to an ICU. Ample evidence demonstrates that injured elderly patients are less likely to receive care at trauma centers despite ample evidence that they are at increased risk for adverse outcomes after injury because of limited cardiovascular reserve, comorbidities, and general frailty.[5][8][12][15–37]
A retrospective analysis of 10 years (1995–2004) of the Maryland Ambulance Information System by Chang et al[16] in 2008 found that among 26,565 patients, the risk for undertriage was significantly higher among those older than 65 years (49.9 vs. 17.8%; p < 0.001).
Furthermore, on multivariate analysis (controlling for year, sex, physiology, injury, mechanism, transport reasons, emergency medical service provider level training, presence or absence of specific injuries, and jurisdictional region), aged 65 years or older emerged as an independent risk factor for undertriage (odds ratio, 0.48 [range, 0.3–0.76]) with inadequate training, unfamiliarity with protocol, and possible age bias listed by survey respondents as common reasons for not bringing elderly patients to trauma centers. The previous version of this PMG and subsequent literature have demonstrated the fact that a large proportion of injured elderly patients return to independent living. As such, age should not be used as a sole criterion for limiting care.[38–41]
In comparisons of care at acute care hospitals (versus care at designated trauma centers), elderly patients seem to be less likely to experience preventable adverse events and are more likely to have a lower risk-adjusted mortality if treated at trauma centers and/or hospitals with dedicated surgeon-intensivists.[4][6][42] One large study of risk-adjusted outcomes found that patients younger than 55 years treated at trauma centers were at significantly decreased risk for postinjury mortality (>25% lower), whereas those who were aged 55 years or older experienced no such apparent benefit.
MacKenzie and her coauthors[4] admitted that their study may not be well suited to answer the question as to whether typical “elderly” injured patients should be treated at trauma centers because of the nonstandard age cutoff and the low numbers of severely injured elderly patients in their sample. One piece of evidence supporting the benefit of triage to designated trauma centers was published by Meldon et al.[5] in 2002 and included risk-adjusted assessment of outcomes for a population of patients aged 80 years or older. In this evaluation, outcomes varied between designated trauma centers and other nondesignated acute care settings. Not surprisingly, head injury, injury severity, and lack of trauma center verification are associated with hospital mortality in very elderly trauma patients.[7][43][44]
Data from a well-executed single-center study demonstrated more than 30% increase in risk-adjusted survival for elderly patients after initiating age 70 years or older as an indicator for trauma alert in a busy urban trauma center.[17] Patients in this sample also were reported to have received liberal application of ICU care and invasive monitoring. As such, we cannot yet determine which of these three interventions yielded the improved survival; it would seem prudent, however, to have a lower threshold for early aggressive evaluation and treatment until multicenter controlled trial data become available. However, it must also be acknowledged that elderly patients with severe traumatic brain injury (sustained GCS score <9) have at least an 80% likelihood of death or long-term placement as their discharge destination, thus justifying discussions regarding goals of care if no improvement in GCS is seen after the initial phase of care and after withdrawal of all sedatives.[29]
This update group is not able to carry forward the previous versions’ recommendations regarding the need to prevent complications because it seems nearly impossible to implement given the universal imperative to prevent complications.[45] Likewise, previous assertions that elderly patients with low revised trauma scores, GCS scores, and respiratory rate on presentation have 100% mortality no longer seem relevant in an era when many more patients are receiving prehospital sedation, muscle relaxants, and intubation.
Correction of Anticoagulation
Increasing numbers of elderly Americans take anticoagulants and antiplatelet agents for a variety of indications. Although these agents have proven overall benefit for patients at risk for thrombotic or embolic events, these medications increase the risk for postinjury hemorrhage and alterations in the postdischarge destination.[46–56]
Substantial variation exists in practice patterns related to correction of iatrogenic and therapeutic coagulopathy after injury despite the work of Ivascu et al.,[57][58] which demonstrated a more than 75% decrease in mortality related to posttraumatic intracranial hemorrhage in elderly patients with Coumadin-related coagulopathy after implementation of a protocol to ensure rapid head computed tomography, initiation of INR-correcting therapy within 1.9 hours, and full correction of coagulopathy within 4 hours of admission.[57–59] The same authors suggested that reversal of INR is not necessary in the absence of intracranial bleeding. The degree of correction indicated in elderly patients with intracranial bleeding is not completely clear, but several authors have concluded that INR should be rapidly corrected to a value of less than 1.6 with fresh-frozen plasma (15 mg/kg or ∼4 units) and vitamin K IV.[57][60] Those who stop their Coumadin-based anticoagulation after injury are at lower risk for major hemorrhage after discharge but at increased risk for thromboembolism.[61]
Little is known regarding the optimal means for correcting iatrogenic platelet dysfunction in injured patients, although it seems clear that patients taking antiplatelet agents are at an increased risk for postinjury hemorrhage.[48][49][51][60][62–66]
End Points of Resuscitation
The previous version of this guideline advocated the near-ubiquitous use of Swan-Ganz catheters in moderately to severely injured elderly patients followed by optimization of cardiac output and oxygen delivery variables to supratherapeutic values.[38] Whereas it remains clear that younger patients progressively increase their cardiac index and oxygen delivery following multiple trauma, elderly patients begin with low levels that often fail to increase.[67]
In one large multicenter examination of “dry” versus “wet” classes of resuscitation in critically ill patients (not exclusively in elderly or injured patients), there was no difference in survival.[68]
There was, however, a marginal increase in ventilator-free days in the conservative fluid group without increased risk for dialysis, pointing to the possibility that injured patients might also fare better if fluid management focused more on the pulmonary effects than the theoretical benefits to renal perfusion.
In other well-performed retrospective analyses not performed in elderly patients, multiple authors have described augmentation of postinjury oxygen delivery (to >500), yielding an increased risk for intra-abdominal hypertension, compartment syndrome, and death, with no survival benefit (odds ratio, 0.86; range, 0.6–1.2).[45][69]Base deficit values of −6 mEq/L or less are markers of severe injury and significant mortality in all trauma patients but especially in the elderly in which this value may predict as much as a 60% risk for mortality as compared with those with a base deficit of −5 mEq/L or higher who have less than 23% risk for mortality.[70]Admittedly, Davis and Kaups[70] made the elderly assignment at age 55 years rather than 65 years, but we suspect that had they chosen the older cutoff for age (≥65 years), the differentiating effect would be yet more dramatic.
Summary
In the relative absence of data to the contrary, our elderly patients should receive care at centers that have devoted specific resources to attaining excellence in the care of the injured using similar criteria to those used in younger patients. Preexisting conditions and/or severe anatomic injuries dramatically increase the risk of poor outcome in elderly patients.
Age and anticoagulants and antiplatelet agents increase the risk for postinjury hemorrhage and require assessment of coagulation profile swiftly following admission. Base deficit (−6 mEq/L or less) is a marker of severe injury and significant mortality in all trauma patients and should be used in consideration for ICU admission. A Glasgow Coma Scale score of 8 or less, remaining low after 72 hours, provides important information regarding long-term prognosis.
Future Investigations
Potentially useful areas for future study identified by this guideline include the following items:
- Creation of robust predictive models to facilitate quality/performance improvement in elderly populations are needed, especially as such efforts pertain to triage decisions regarding invasive monitoring and aggressiveness of care.
- A deeper understanding is needed as to when exactly “elderly” status begins physiologically.
- More insight is needed as to whether medication-induced platelet dysfunction requires correction with the same urgency as warfarin-induced coagulopathy.
- Little is known regarding how we should address next-generation oral anticoagulants that cannot be corrected with blood products or pharmaceuticals. Can this be accomplished in an effective and cost-efficient manner?
- Finally, can we accommodate the ever-increasing volume of elderly patients coming to our trauma centers (in addition to our current patient volumes) while maintaining high standards of care and avoiding mercenary triage decisions?
Authorship
J.F.C. designed this study. J.F.C., A.M.I., G.T.M., R.D.B., and C.I.S. reviewed the evidence. All authors contributed to the preparation and editing of the manuscript. J.F.C. and T.S. created the figures and tables.
Disclosure
The authors declare no conflicts of interest.
References
- Merriam-Webster Online Dictionary. Available at: http://www.merriam-webster.com. Accessed 5 October 2012.
- Camilloni L, Farchi S, Giorgi Rossi P, et al.. Mortality in elderly injured patients: the role of comorbidities. Int J Inj Contr Saf Promot. 2008; 15: 25–31.
- Grossman MD, Miller D, Scaff DW, et al.. When is an elder old? Effect of preexisting conditions on mortality in geriatric trauma. J Trauma. 2002; 52: 242–246.
- MacKenzie EJ, Rivara FP, Jurkovich GJ, et al.. A national evaluation of the effect of trauma-center care on mortality. New Engl J Med. 2006; 354: 366–378.
- Meldon SW, Reilly M, Drew BL, et al.. Trauma in the very elderly: a community-based study of outcomes at trauma and nontrauma centers. J Trauma. 2002; 52: 79–84.
- Nathens AB, Rivara FP, MacKenzie EJ, et al.. The impact of an intensivist-model ICU on trauma-related mortality. Ann Surg. 2006; 244: 545–554.
- Rzepka SG, Malangoni MA, Rimm AA. Geriatric trauma hospitalization in the United States: a population-based study. J Clin Epidemiol. 2001; 54: 627–633.
- Tepas JJ 3rd, Veldenz HC, Lottenberg L, et al.. Elderly injury: a profile of trauma experience in the Sunshine (Retirement) State. J Trauma. 2000; 48: 581–584; discussion 584–586.
- Scheetz LJ. Effectiveness of prehospital trauma triage guidelines for the identification of major trauma in elderly motor vehicle crash victims. J Emerg Nurs. 2003; 29: 109–115.
- McGwin G, Jr, May AK, Melton SM, et al.. Recurrent trauma in elderly patients. Arch Surg. 2001; 136: 197–203.
- Mann NC, Cahn RM, Mullins RJ, et al.. Survival among injured geriatric patients during construction of a statewide trauma system. J Trauma. 2001; 50: 1111–1116.
- Grant PT, Henry JM, McNaughton GW. The management of elderly blunt trauma victims in Scotland: evidence of ageism? Injury. 2000; 31: 519–528.
- Simon BJ, Cushman J, Barraco R, et al.. Pain management guidelines for blunt thoracic trauma. J Trauma. 2005; 59: 1256–1267.
- Eastern Association for the Surgery of Trauma (EAST) Ad Hoc Committee on Practice Management Guideline Development. Utilizing evidence based outcome measures to develop practice management guidelines: a primer.EAST; 2000. Available at: http://www.east.org/tpg/primer.pdf.
- Bergeron E, Lavoie A, Clas D, et al.. Elderly trauma patients with rib fractures are at greater risk of death and pneumonia. J Trauma. 2003; 54: 478–485.
- Chang DC, Bass RR, Cornwell EE, et al.. Undertriage of elderly trauma patients to state-designated trauma centers. Arch Surg. 2008; 143: 776–781; discussion 782.
- Demetriades D, Karaiskakis M, Velmahos G, et al.. Effect on outcome of early intensive management of geriatric trauma patients. Br J Surg. 2002; 89: 1319–1322.
- Demetriades D, Sava J, Alo K, et al.. Old age as a criterion for trauma team activation. J Trauma. 2001; 51: 754–756; discussion 756–757.
- Ma MH, MacKenzie EJ, Alcorta R, et al.. Compliance with prehospital triage protocols for major trauma patients. J Trauma. 1999; 46: 168–175.
- Pracht EE, Langland-Orban B, Tepas JJ 3rd, et al.. Analysis of trends in the Florida Trauma System (1991–2003): changes in mortality after establishment of new centers. Surgery. 2006; 140: 34–43.
- Sharma OP, Oswanski MF, Sharma V, et al.. An appraisal of trauma in the elderly. Am Surg. 2007; 73: 354–358.
- Alexander JQ, Gutierrez CJ, Mariano MC, et al.. Blunt chest trauma in the elderly patient: how cardiopulmonary disease affects outcome. Am Surg. 2000; 66: 855–857.
- Belzberg H, Wo CC, Demetriades D, et al.. Effects of age and obesity on hemodynamics, tissue oxygenation, and outcome after trauma. J Trauma. 2007; 62: 1192–1200.
- Bergeron E, Clement J, Lavoie A, et al.. A simple fall in the elderly: not so simple. J Trauma. 2006; 60: 268–273; discussion 1046–1047.
- Bulger EM, Arneson MA, Mock CN, et al.. Rib fractures in the elderly. J Trauma. 2000; 48: 1040–1046.
- Mitra B, Cameron PA, Gabbe BJ, et al.. Management and hospital outcome of the severely head injured elderly patient. ANZ J Surg. 2008; 78: 588–592.
- Mohindra S, Mukherjee KK, Gupta R, et al.. Continuation of poor surgical outcome after elderly brain injury. Surg Neurol. 2008; 69: 474–477.
- Sharma OP, Oswanski MF, Jolly S, et al.. Perils of rib fractures. Am Surg. 2008; 74: 310–314.
- LeBlanc J, de Guise E, Gosselin N, et al.. Comparison of functional outcome following acute care in young, middle-aged and elderly patients with traumatic brain injury. Brain Inj. 2006; 20: 779–790.
- Yilmaz S, Karcioglu O, Sener S. The impact of associated diseases on the etiology, course and mortality in geriatric trauma patients. Eur J Emerg Med. 2006; 13: 295–298.
- Gallagher SF, Williams B, Gomez C, et al.. The role of cardiac morbidity in short- and long-term mortality in injured older patients who survive initial resuscitation. Am J Surg. 2003; 185: 131–134.
- O’Brien D P, Luchette FA, Pereira SJ, et al.. Pelvic fracture in the elderly is associated with increased mortality. Surgery. 2002; 132: 710–714; discussion 714–715.
- Taylor MD, Tracy JK, Meyer W, et al.. Trauma in the elderly: intensive care unit resource use and outcome. J Trauma. 2002; 53: 407–414.
- Susman M, DiRusso SM, Sullivan T, et al.. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J Trauma. 2002; 53: 219–223; discussion 223–214.
- Sterling DA, O’Connor JA, Bonadies J. Geriatric falls: injury severity is high and disproportionate to mechanism. J Trauma. 2001; 50: 116–119.
- Ferrera PC, Bartfield JM, D’Andrea CC. Outcomes of admitted geriatric trauma victims. Am J Emerg Med. 2000; 18: 575–580.
- McGwin G Jr, Melton SM, May AK, et al.. Long-term survival in the elderly after trauma. J Trauma. 2000; 49: 470–476.
- Jacobs DG, Plaisier BR, Barie PS, et al.. Practice management guidelines for geriatric trauma: the EAST Practice Management Guidelines Work Group. J Trauma. 2003; 54: 391–416.
- Albrecht RM, Schermer CR, Morris A. Nonoperative management of blunt splenic injuries: factors influencing success in age >55 years. Am Surg. 2002; 68: 227–230; discussion 230–221.
- Damadi AA, Saxe AW, Fath JJ, et al.. Cervical spine fractures in patients 65 years or older: a 3-year experience at a level I trauma center. J Trauma. 2008; 64: 745–748.
- Roth BJ, Velmahos GC, Oder DB, et al.. Penetrating trauma in patients older than 55 years: a case-control study. Injury. 2001; 32: 551–554.
- Gowing R, Jain MK. Injury patterns and outcomes associated with elderly trauma victims in Kingston, Ontario. Can J Surg. 2007; 50: 437–444.
- Lawes D. A retrospective review of emergency admission for head injury in the over 75s. Injury. 2002; 33: 349–351.
- Mosenthal AC, Lavery RF, Addis M, et al.. Isolated traumatic brain injury: age is an independent predictor of mortality and early outcome. J Trauma. 2002; 52: 907–911.
- Balogh Z, McKinley BA, Cocanour CS, et al.. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg. 2003; 138: 637–642; discussion 642–633.
- Bouras T, Stranjalis G, Korfias S, et al.. Head injury mortality in a geriatric population: differentiating an “edge” age group with better potential for benefit than older poor-prognosis patients. J Neurotrauma. 2007; 24: 1355–1361.
- Cohen DB, Rinker C, Wilberger JE. Traumatic brain injury in anticoagulated patients. J Trauma. 2006; 60: 553–557.
- Lavoie A, Ratte S, Clas D, et al.. Preinjury warfarin use among elderly patients with closed head injuries in a trauma center. J Trauma. 2004; 56: 802–807.
- Mina AA, Bair HA, Howells GA, et al.. Complications of preinjury warfarin use in the trauma patient. J Trauma. 2003; 54: 842–847.
- Mina AA, Knipfer JF, Park DY, et al.. Intracranial complications of preinjury anticoagulation in trauma patients with head injury. J Trauma. 2002; 53: 668–672.
- Karni A, Holtzman R, Bass T, et al.. Traumatic head injury in the anticoagulated elderly patient: a lethal combination. Am Surg. 2001; 67: 1098–1100.
- Kennedy DM, Cipolle MD, Pasquale MD, et al.. Impact of preinjury warfarin use in elderly trauma patients. J Trauma. 2000; 48: 451–453.
- Kirsch MJ, Vrabec GA, Marley RA, et al.. Preinjury warfarin and geriatric orthopedic trauma patients: a case-matched study. J Trauma. 2004; 57: 1230–1233.
- Wojcik R, Cipolle MD, Seislove E, et al.. Preinjury warfarin does not impact outcome in trauma patients. J Trauma. 2001; 51: 1147–1151; discussion 1151–1142.
- Franko J, Kish KJ, O’Connell BG, et al.. Advanced age and preinjury warfarin anticoagulation increase the risk of mortality after head trauma. J Trauma. 2006; 61: 107–110.
- Gage BF, Birman-Deych E, Kerzner R, et al.. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005; 118: 612–617.
- Ivascu FA, Howells GA, Junn FS, et al.. Rapid warfarin reversal in anticoagulated patients with traumatic intracranial hemorrhage reduces hemorrhage progression and mortality. J Trauma. 2005; 59: 1131–1137; discussion 1137–1139.
- Ivascu FA, Janczyk RJ, Junn FS, et al.. Treatment of trauma patients with intracranial hemorrhage on preinjury warfarin. J Trauma. 2006; 61: 318–321.
- Coimbra R, Hoyt DB, Anjaria DJ, et al.. Reversal of anticoagulation in trauma: a North American survey on clinical practices among trauma surgeons. J Trauma. 2005; 59: 375–382.
- McMillian WD, Rogers FB. Management of prehospital antiplatelet and anticoagulant therapy in traumatic head injury: a review. J Trauma. 2009; 66: 942–950.
- Hackam DG, Kopp A, Redelmeier DA. Prognostic implications of warfarin cessation after major trauma: a population-based cohort analysis. Circulation. 2005; 111: 2250–2256.
- Williams TM, Sadjadi J, Harken AH, et al.. The necessity to assess anticoagulation status in elderly injured patients. J Trauma. 2008; 65: 772–776; discussion 776–777.
- Pieracci FM, Eachempati SR, Shou J, et al.. Degree of anticoagulation, but not warfarin use itself, predicts adverse outcomes after traumatic brain injury in elderly trauma patients. J Trauma. 2007; 63: 525–530.
- Pieracci FM, Eachempati SR, Shou J, et al.. Use of long-term anticoagulation is associated with traumatic intracranial hemorrhage and subsequent mortality in elderly patients hospitalized after falls: analysis of the New York State Administrative Database. J Trauma. 2007; 63: 519–524.
- Wong DK, Lurie F, Wong LL. The effects of clopidogrel on elderly traumatic brain injured patients. J Trauma. 2008; 65: 1303–1308.
- Ohm C, Mina A, Howells G, et al.. Effects of antiplatelet agents on outcomes for elderly patients with traumatic intracranial hemorrhage. J Trauma. 2005; 58: 518–522.
- Epstein CD, Peerless J, Martin J, et al.. Oxygen transport and organ dysfunction in the older trauma patient. Heart Lung. 2002; 31: 315–326.
- Stewart RM, Park PK, Hunt JP, et al.. Less is more: improved outcomes in surgical patients with conservative fluid administration and central venous catheter monitoring. J Am Coll Surg. 2009; 208: 725–735; discussion 735–727.
- Heyland DK, Cook DJ, King D, et al.. Maximizing oxygen delivery in critically ill patients: a methodologic appraisal of the evidence. Crit Care Med. 1996; 24: 517–524.
- Davis JW, Kaups KL. Base deficit in the elderly: a marker of severe injury and death. J Trauma. 1998; 45: 873–877.