Antimotility Agents for the Treatment of Acute Noninfectious Diarrhea in Critically Ill Patients
Published 2019
Citation: J Trauma. 87(4):915-921, October 2019
Authors
Bugaev, Nikolay MD; Bhattacharya, Bishwajit MD; Chiu, William C. MD; Como, John J. MD, MPH; Cripps, Michael W. MD; Ferrada, Paula MD; Gelbard, Rondi B. MD; Gondek, Stephen MD, MPH; Kasotakis, George MD, MPH; Kim, Dennis MD; Mentzer, Caleb DO; Robinson, Bryce R. H. MD; Salcedo, Edgardo S. MD; Yeh, D. Dante MD
Author Information
From the Division of Trauma & Acute Care Surgery (N.B.), Tufts Medical Center, Tufts University School of Medicine. Boston, Massachusetts; Department of Surgery (B.B.), Yale School of Medicine, New Haven, Connecticut; Department of Surgery (W.C.C.), University of Maryland School of Medicine, R Adams Cowley Shock Trauma Center, University of Maryland Medical Center, Baltimore, Maryland; Department of Surgery (J.J.C.), MetroHealth Medical Center, Cleveland, Ohio; Trauma Division of General and Acute Care Surgery, Department of Surgery (M.W.C.), The University of Texas Southwestern Medical Center at Dallas, Texas; Virginia Commonwealth University (P.F.), Richmond, Virginia; Emory University School of Medicine (R.B.G.), Department of Surgery, Division of Trauma and Surgical Critical Care, Grady Memorial Hospital, Atlanta, Georgia; Division of Trauma and Surgical Critical Care (S.G.), Vanderbilt University Medical Center, Nashville, Tennessee; Division of Trauma and Critical Care Surgery, Department of Surgery (G.K.), Duke University School of Medicine, Durham, North Carolina; Division of Trauma/Acute Care Surgery/Surgical Critical Care (D.K.), LA County Harbor-UCLA Medical Center, Torrance, California; Division of Trauma, Critical Care, & Acute Care Surgery (C.M.), Spartanburg Medical Center, Spartanburg, South Carolina; Harborview Medical Center (B.R.H.R.), University of Washington, Seattle, Washington; Division of Trauma, Acute Care Surgery and Surgical Critical Care, Department of Surgery (E.S.S.), University of California Davis, Sacramento, California; and University of Miami (D.D.Y.), Ryder Trauma Center, Miami, Florida.
Submitted: June 12, 2019, Accepted: June 20, 2019, Published online: July 19, 2019.
This article was presented as a podium presentation at the 32nd EAST Annual Scientific Assembly on January 15 to 19, 2019, at the JW Marriott Austin in Austin, Texas.
Address for reprints: Nikolay Bugaev, MD, Division of Trauma & Acute Care Surgery, Tufts Medical Center, Tufts University School of Medicine, Boston, MA, 800 Washington St, 4488, Boston, MA 0211; email: nbugaev@tuftsmedicalcenter.org.
Online date: July 22, 2019
BACKGROUND Acute noninfectious diarrhea is a common phenomenon in intensive care unit patients. Multiple treatments are suggested but the most effective management is unknown. A working group of the Eastern Association for the Surgery of Trauma, aimed to evaluate the effectiveness of loperamide, diphenoxylate/atropine, and elemental diet on acute noninfectious diarrhea in critically ill adults and to develop recommendations applicable to daily clinical practice.
METHODS The literature search identified 11 randomized controlled trials (RCT) appropriate for inclusion. The Grading of Recommendations Assessment, Development, and Evaluation methodology was applied to evaluate the effect of loperamide, diphenoxylate/atropine, and elemental diet on the resolution of noninfectious diarrhea in critically ill adults based on selected outcomes: improvement in clinical diarrhea, fecal frequency, time to the diarrhea resolution, and hospital length of stay.
RESULTS The level of evidence was assessed as very low. Analyses of 10 RCTs showed that loperamide facilitates resolution of diarrhea. Diphenoxylate/atropine was evaluated in three RCTs and was as effective as loperamide and more effective than placebo. No studies evaluating elemental diet as an intervention in patients with diarrhea were found.
CONCLUSION Loperamide and diphenoxylate/atropine are conditionally recommended to be used in critically ill patients with acute noninfectious diarrhea.
LEVEL OF EVIDENCE Systematic Review/Guidelines, level III.
Acute noninfectious diarrhea is a frequent phenomenon in critically ill patients with a reported prevalence of up to 78%.[1] The etiology of diarrhea in intensive care unit (ICU) settings is multifactorial and includes disease-related, medication-related, feeding-related, infectious, and noninfectious causes.[2] The occurrence of diarrhea in critically ill patients is associated with increased ICU length of stay, morbidity, and mortality.[3]
The management of noninfectious diarrhea in critical illness is directed toward treatment of the underlying cause, fluid-electrolyte correction, avoidance of medications associated with diarrhea[1][2] and antidiarrheal agents. Several antidiarrheal medications (loperamide, probiotics, prebiotics, cholestyramine) have been proposed for treating diarrhea in critically ill patients.[2] In the non-ICU setting, treatment with simethicone,[4][5]Saccharomyces boulardii,[6] bismuth subsalicylate,[7] diphenoxylate/atropine,[8][9] and wood creosote[10] has been reported. Outside of the United States, additional agents include: racecadotril,[11] activated attapulgite,[12] and hydrated aluminum magnesium silicate.[13] The goal of this review is to evaluate the existing evidence and create recommendations regarding the routine use of loperamide, diphenoxylate/atropine, and elemental diet in critically ill adults with acute noninfectious diarrhea.
Objectives
The objective of this review was to evaluate the effect of loperamide, diphenoxylate/atropine, and elemental diet on the resolution of acute noninfectious diarrhea in adult (age >18 years) critically ill patients using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.[14]
Through an iterative voting process, the EAST diarrhea practice management workgroup developed the following PICO questions (Population, Intervention, Comparison, Outcomes):
PICO 1
- In critically ill adults with acute noninfectious diarrhea (P), should treatment with loperamide be administered (I) compared with usual care without loperamide (C) to improve clinical diarrhea, fecal frequency, and time to diarrhea resolution (O)?
PICO 2
- In critically ill adults with acute noninfectious diarrhea (P), should treatment with diphenoxylate/atropine be administered (I) compared to usual care without diphenoxylate/atropine (C) to improve clinical diarrhea, fecal frequency, and time to diarrhea resolution (O)?
PICO 3
- In critically ill adults with acute noninfectious diarrhea (P), should treatment with semi- or full-elemental tube feeds be administered (I) compared to usual care without semi- or full-elemental tube feeds (C) to improve clinical diarrhea, fecal frequency, and time to diarrhea resolution (O)?
Outcome Measure Type
The members of the working group proposed outcomes related to resolution of diarrhea. All outcomes were independently rated by each group member on a scale from 1 to 9, and the median score for each outcome was calculated and assigned as the final score.
Outcomes scored between 7 and 9 were considered critical and included: improved clinical diarrhea defined as resolution of either bloating, abdominal pain, stool consistency, or self-reported improvement; fecal frequency, and time to diarrhea resolution.
Hospital length of stay was also considered a critical outcome, but none of the selected studies reported it.
Identification of References
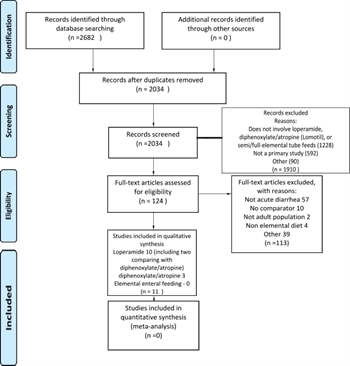
A medical librarian performed a search of citations in the following databases: PubMed, CINAHIL, Embase, Cochrane Library, Web of Science, PROSPERO, RefWorks, and Scopus. The search was performed using the following MeSH terms: “Diarrhea,” “Loperamide,” “Atropine sulfate-diphenoxylate hydrochloride drug combination,” “Atropine,” “Diphenoxylate,” “Lomotil,” “Lonox,” “Lomocot,” “Imodium,” “Diamode,” “Imogen,” “Imotil,” “Imperim,” “Kaodene,” “Kao Pave,” “Enteral Nutrition,” “Elemental,” “Semi elemental,” “Monomeric,” and “Oligomeric.” The search time period was from January 1, 1965, to February 28, 2017. An updated search was performed from February 28, 2017, to February 28, 2019.
Original clinical retrospective studies, prospective observational studies, and randomized controlled trials (RCT) in adults (age, ≥18 years) were considered for inclusion. Review articles, meta-analyses, case reports, case series without a comparison group, manuscripts that evaluated colonic pseudo-obstruction, and non-English language publications were excluded.
Two members of the working group independently screened titles and abstracts of the selected references. Next, full text articles were independently screened by two independent working group members. Only studies describing management of acute noninfectious diarrhea were included. Studies describing chronic diarrhea, either radiation- or chemotherapy-induced diarrhea, inflammatory bowel disease-related diarrhea, high-output ileostomy, and diarrhea after bowel resection were excluded. Selected studies were included for final data extraction and analysis. No studies describing selected interventions in critically ill adults with acute noninfectious diarrhea were found. All included studies contained patients with noninfectious acute diarrhea who were managed as an outpatient.
At each stage of the screening process, disagreements between the reviewers were adjudicated by discussion and consensus among the individuals. When consensus was not reached, a third reviewer was involved as an arbitrator (Fig. 1). No external funding was obtained for this work.
Data Extraction and Methodology
A total of 11 RCTs were included.[4–10][15–18] The data were independently extracted by two authors and entered into a Microsoft Excel (Richmond, WA) spreadsheet. All time-related outcomes were presented in hours.
No studies addressing the role of elemental diet in the treatment of diarrhea were found.
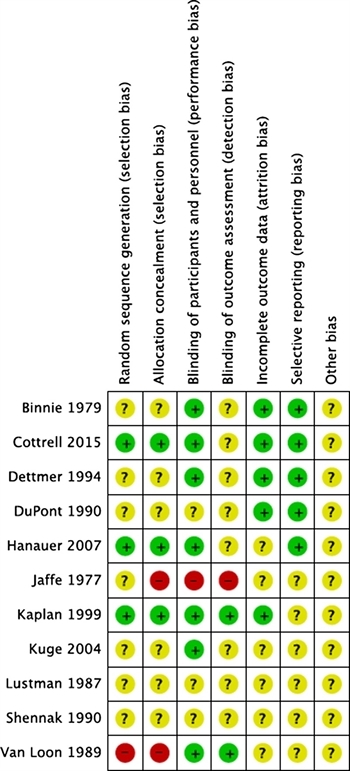
Risk of Bias
Risk of bias of the included RCTs was assessed in six domains: sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and “other issues” (Fig. 2).
Grading of Evidence
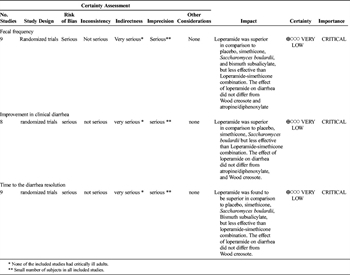
The available evidence was assessed according to the GRADE methodology as high, moderate, low, or very low quality. The quality of evidence was downgraded for study design, risk of bias, inconsistency, indirectness, and imprecision.[14]
Results for the Use of Loperamide (PICO 1)
In critically ill adults with acute noninfectious diarrhea (P), should treatment with loperamide be administered (I) compared to usual care without loperamide (C) to improve clinical diarrhea, fecal frequency, and time to diarrhea resolution?
Qualitative Analysis
There were a total of 10 RCTs[4–10][15–17] that described 2,325 patients, including 819 patients who received loperamide. Loperamide was compared with placebo,[4][5][15–17] a combination of loperamide-simethicone,[4][5] simethicone alone,[4][5]Saccharomyces boulardii,[6] bismuth subsalicylate,[7] diphenoxylate/atropine,[8][9] and wood creosote.[10]
The effect of loperamide compared to placebo was evaluated in five studies.[4][5][15–17] The fecal frequency improved: every 12 hours during the first 2 days;[4][5] at the end of the first 24 hours;[16][17] and 2 days[17] since the beginning of the treatment with loperamide. Diarrhea-related symptoms improved faster in the loperamide group,[15][16] as well as the stool consistency.[16] Time to the diarrhea resolution was faster with loperamide.[4][5][15][16]
A combination of loperamide with simethicone compared to loperamide alone in acute diarrhea showed overall decreased number of bowel movements in the first 48 hours, faster relief of diarrhea-related symptoms and faster resolution of the diarrhea.[4][5] The same studies evaluating effect of simethicone alone compared with loperamide and found that loperamide was more effective in all selected outcomes. Time to diarrhea resolution was significantly shorter in the loperamide group.[4][5][15][16] All included studies showed a beneficial effect of loperamide on acute diarrhea in comparison to placebo.
Loperamide was compared with the combination of loperamide with simethicone and simethicone alone.[4][5] Loperamide was found to be more effective when compared with simethicone alone, but loperamide-simethicone was more beneficial than loperamide in terms of fecal frequency, time to diarrhea resolution, and overall improvement diarrhea-related symptoms.
Two studies evaluated performance of loperamide compared with diphenoxylate/atropine.[8][9] Both studies showed a positive effect of loperamide and diphenoxylate/atropine on stool frequency,[8] time to diarrhea resolution,[8][9] and improvement in clinical diarrhea,[8][9] but overall, no significant difference was found between the two medications.
Cottrel et al.[6] showed that the loperamide-simethicone combination decreased fecal frequency and time to diarrhea resolution in comparison to the probiotic Saccharomyces boulardii. Loperamide was more effective in comparison to bismuth subsalicylate.[7] No differences in all selected outcomes were found between loperamide and wood creosote.[10]
Quantitative Analysis
No data allowing quantitative analysis were available, as the outcomes of interests, improvement of clinical diarrhea, fecal frequency, and time to diarrhea resolution, were reported in different time frames and severity scales, which precluded performing such analysis.
Grading the Evidence
The evidence was assessed applying the GRADE framework (Table 1). The level of evidence was downgraded for indirectness since none of the included studies described the effect of loperamide on critically ill patients. The level of evidence was further decreased for imprecision as most of the included studies described small cohorts.
The bias assessment revealed that in most of the included RCTs, sequence generation and allocation concealment (selection bias) were not clearly described, and there was lack of blinding of either participant or the research staff to the group assignments (performance bias) (Fig. 1). Because of these factors, the quality of evidence was deemed to be very low.
Recommendations for the Use of Loperamide (PICO 1)
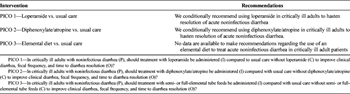
Based on qualitative analysis of the very low level of evidence, we conditionally recommend administering loperamide in critically ill adults to improve clinical diarrhea, fecal frequency, and time to diarrhea resolution (Table 3).
Results for the Use of Diphenoxylate/Atropine (PICO 2)
In critically ill adults with noninfectious diarrhea (P), should treatment with diphenoxylate/atropine be administered (I) compared with usual care without diphenoxylate/atropine (C) to improve clinical diarrhea, fecal frequency, and time to diarrhea resolution?
Qualitative Analysis
There were three RCT studies evaluating the effect of diphenoxylate/atropine on diarrhea. Lustman et al. compared diphenoxylate/atropine with placebo and found faster resolution of diarrhea in the diphenoxylate/atropine patients.[18] Two other studies compared diphenoxylate/atropine with loperamide and were described in the PICO 1.[8][9] Both medications were effective in resolving diarrhea, but no differences between them were found.
Quantitative Analysis
No data allowing quantitative analysis were available.
Grading the Evidence
The evidence was assessed applying the GRADE framework (Table 2). The level of evidence was downgraded for indirectness since none of the included studies described effect of diphenoxylate/atropine on critically ill patients. The level of evidence was further decreased for imprecision as all of the included studies described small cohorts. The bias assessment revealed that all of the included RCTs had lack of blinding of either participant or the research staff to the group assignments (performance bias). Also, sequence generation and allocation concealment (selection bias) were not clearly described in all included studies (Fig. 1). Because of these factors, the quality of evidence was deemed to be very low.
Recommendations for the Use of Atropine/Diphenoxylate (PICO 2)
Based on qualitative analysis of the very low level of evidence, we conditionally recommend administering diphenoxylate/atropine in critically ill adults to improve clinical diarrhea, fecal frequency, and time to diarrhea resolution (Table 3).
Results for the Use of Elemental Diet (PICO 3)
In critically ill adults with noninfectious diarrhea (P), should treatment with semi- or full-elemental tube feeds be administered (I) compared to usual care without semi- or full-elemental tube feeds (C) to improve clinical diarrhea, fecal frequency, and time to diarrhea resolution (O)?
No studies addressing a role of elemental diet in the management of diarrhea in critically ill patients were found.
Recommendations for the Use of Elemental Diet (PICO 3)
Since no studies addressing a role of elemental diet in the management of diarrhea in critically ill patients were found, we cannot make recommendations regarding the use of an elemental diet to treat diarrhea in critically ill adult patients.
Using These Guidelines in Clinical Practice
This systematic review evaluated the role of loperamide and diphenoxylate/atropine in the management of noninfectious diarrhea in critically ill adult patients. Both agents are effective in facilitating the resolution of the diarrhea and can be considered as adjuncts to the diarrhea treatment that also includes elimination of the cause of the diarrhea, and correction of any fluid-electrolyte imbalances. This conclusion was made based on very low level of evidence, as none of the included studies reported the usage of these two agents in ICU patients but only ambulatory patients.
Two of the included studies directly compared loperamide and diphenoxylate/atropine[8][9] and found that both agents were effective, but neither agent was superior to the other. The potential patient-related considerations, including the medication cost, number of pills required to achieve a therapeutic effect, and the risk of adverse events were very similar between these two medication regimens. Therefore, we cannot make recommendations regarding a preferable usage of loperamide over diphenoxylate/atropine.
Future Research
Our systematic review aimed to evaluate a role of loperamide and diphenoxylate/atropine in the management of acute noninfectious diarrhea in critically ill adults. The extensive literature search did not yield any studies evaluating the usage of these two agents in critically ill patients with acute noninfectious diarrhea, but only in the outpatient setting. Given the frequent occurrence of diarrhea in critical illness, morbidity associated with it, and lack of a single effective agent to treat the diarrhea, the need for studies assessing the role of loperamide and diphenoxylate/atropine management is clearly evident. The lack of publications about these two agents provides multiple scientific opportunities. As the first step, descriptive studies addressing usage of these two agents in critically ill patients will provide a starting point for future investigations.
Conclusion
Loperamide and diphenoxylate/atropine should be considered in the management of critically ill patients with acute noninfectious diarrhea.
Authorship
N.B. participated in the study design, literature search, data collection, data analysis, data interpretation, drafting the article. B.B. participated in the study design, data collection, data interpretation, and critical revisions. W.C. participated in the study design, data collection, data interpretation, and critical revisions. J.C. participated in the study design, data collection, data interpretation, and critical revisions. M.C. participated in the study design, data collection, data interpretation, and critical revisions. P.F. participated in the study design, data collection, data interpretation, and critical revisions. R.G. participated in the study design, data collection, data interpretation, and critical revisions. S.G. participated in the study design, data collection, data interpretation, and critical revisions. G.K. participated in the study design, data collection, data interpretation, and critical revisions. D.K. participated in the study design, data collection, data interpretation, and critical revisions. C.M. participated in the study design, data collection, data interpretation, and critical revisions. B.R. participated in the study design, data collection, data interpretation, and critical revisions. E.S. participated in the study design, data collection, data interpretation, and critical revisions. D.D.Y. participated in the study idea, study design, literature search, data collection, data analysis, data interpretation, and critical revisions.
Disclosure
The authors declare no funding of conflicts of interest.
References
- Hay T, Bellomo R, Rechnitzer T, See E, Ali Abdelhamid Y, Deane AM. Constipation, diarrhea, and prophylactic laxative bowel regimens in the critically ill: a systematic review and meta-analysis. J Crit Care. 2019. [Epub ahead of print].
- Blaser RA, Deane AM, Fruhwald S. Diarrhoea in the critically ill. Curr Opin Crit Care. 2015;21(2):142–153.
- Tirlapur N, Puthucheary ZA, Cooper JA, Sanders J, Coen PG, Moonesinghe SR, Wilson AP, Mythen MG, Montgomery HE. Diarrhoea in the critically ill is common, associated with poor outcome, and rarely due to Clostridium difficile. Sci Rep. 2016;6:24691.
- Hanauer SB, DuPont HL, Cooper KM, Laudadio C. Randomized, double-blind, placebo-controlled clinical trial of loperamide plus simethicone versus loperamide alone and simethicone alone in the treatment of acute diarrhea with gas-related abdominal discomfort. Curr Med Res Opin. 2007;23(5):1033–1043.
- Kaplan MA, Prior MJ, Ash RR, McKonly KI, Helzner EC, Nelson EB. Loperamide-simethicone vs loperamide alone, simethicone alone, and placebo in the treatment of acute diarrhea with gas-related abdominal discomfort. A randomized controlled trial. Arch Fam Med. 1999;8(3):243–248.
- Cottrell J, Koenig K, Perfekt R, Hofmann R. Comparison of two forms of loperamide-simethicone and a probiotic yeast (Saccharomyces boulardii) in the treatment of acute diarrhoea in adults: a randomised non-inferiority clinical trial; loperamide–simethicone acute diarrhoea study team. Drugs R D. 2015;15(4):363–373.
- DuPont HL, Flores Sanchez J, Ericsson CD, Mendiola Gomez J, DuPont MW, Cruz Luna A, Mathewson JJ. Comparative efficacy of loperamide hydrochloride and bismuth subsalicylate in the management of acute diarrhea. Am J Med. 1990;88(6A):15S–19S.
- Jaffé G. A comparison of lomotil and imodium in acute non-specific diarrhoea. J Int Med Res. 1977;5(3):195–198.
- Binnie IS, Clarke GHR, Cumberbatch JB, et al. A general practice research group. Multicentre general practice comparison of loperamide and diphenoxylate with atropine in the treatment of acute diarrhoea in adults. Br J Clin Pract. 1979;33(3):77–79.
- Kuge T, Shibata T, Willett MS. Multicenter, double-blind, randomized comparison of wood creosote, the principal active ingredient of Seirogan, an herbal antidiarrheal medication, and loperamide in adults with acute nonspecific diarrhea. Clin Ther. 2004;26(10):1644–1651.
- Gebbia V, Carreca I, Testa A, et al. Subcutaneous octreotide versus oral loperamide in the treatment of diarrhea following chemotherapy. Anticancer Drugs. 1993;4(4):443–445.
- DuPont HL, Ericsson CD, DuPont MW, Cruz Luna A, Mathewson JJ. A randomized, open-label comparison of nonprescription loperamide and attapulgite in the symptomatic treatment of acute diarrhea. Am J Med. 1990;88(6A):20S–23S.
- Leber W. A new suspension form of smectite (liquid ‘Diasorb’) for the treatment of acute diarrhoea: a randomized comparative study. Pharmatherapeutica. 1988;5(4):256–260.
- Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, Dahm P. Eastern Association for the Surgery of Trauma Practice Management Guidelines Ad Hoc Committee. The Eastern Association of the Surgery of trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S283–S287.
- Dettmer A. Loperamide oxide in the treatment of acute diarrhea in adults. Clin Ther. 1994;16(6):972–980.
- Shennak MM, Beiruti MA, Ghawi SS. Double-blind comparison of loperamide and placebo against acute diarrhea in adults. Saudi Med J. 1990;11(3):214–217.
- Van Loon FP, Bennish ML, Speelman P, Butler C. Double blind trial of loperamide for treating acute watery diarrhoea in expatriates in Bangladesh. Gut. 1989;30(4):492–495.
- Lustman F, Walters EG, Shroff NE, Akbar FA. Diphenoxylate hydrochloride (Lomotil) in the treatment of acute diarrhoea. Br J Clin Pract. 1987;41(3):648–651.
Keywords:
Diarrhea; loperamide; diphenoxylate/atropine