Blunt Aortic Injury, Evaluation and Management of
Published 2015
Citation: J Trauma. 78(1):136-146, January 2015.
Authors
Fox, Nicole MD; Schwartz, Diane MD; Salazar, Jose H. MD; Haut, Elliott R. MD; Dahm, Philipp MD; Black, James H. MD; Brakenridge, Scott C. MD; Como, John J. MD; Hendershot, Kimberly MD; King, David R. MD; Maung, Adrian A. MD; Moorman, Matthew L. MD; Nagy, Kimberly MD; Petrey, Laura B. MD; Tesoriero, Ronald MD; Scalea, Thomas M. MD; Fabian, Timothy C. MD
Author Information
From the Department of Surgery (N.F.), Cooper University Hospital, Cooper Medical School of Rowan University, Camden, New Jersey; Department of Surgery (D.S.), The Johns Hopkins Bayview Medical Center; Department of Surgery (J.S.O., E.R.H.), The Johns Hopkins School of Medicine; Department of Vascular Surgery and Endovascular Therapy (J.H.B.), The Johns Hopkins Hospital and Johns Hopkins Medical Institutions; and Department of Surgery (R.T., T.M.S.), R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine, Baltimore, Maryland; Department of Urology (P.D.), Malcom Randall VAMC, University of Florida College of Medicine; and Department of Surgery (S.C.B.), University of Florida Health Science Center, UF Health Shands Hospital, Gainesville, Florida; Department of Surgery (J.J.C.), MetroHealth Medical Center; and Division of Acute Care Surgery (M.L.M.), Cleveland Clinic Lerner College of Medicine, Cleveland; and Department of Surgery, Wright State University, Boonshoft School of Medicine, Dayton, Ohio; Department of Surgery (D.R.K.), Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts; Department of Surgery (A.A.M.), Yale University School of Medicine, New Haven, Connecticut; Department of Surgery (K.N.), Cook County Trauma Unit of Stroger Hospital, Chicago, Illinois; Department of Surgery (L.B.P.), Baylor University Medical Center, Dallas, Texas; Department of Surgery (T.C.F.), The University of Tennessee Health Science Center, Memphis, Tennessee.
Submitted: July 7, 2014, Revised: August 3, 2014, Accepted: August 4, 2014.
T.M.S. and T.C.F. should be noted as co-senior authors.
These practice management guidelines were presented at the 26th Annual Scientific Assembly of the Eastern Association for the Surgery of Trauma, January 15–19, 2013, in Scottsdale, Arizona.
Address for reprints: Nicole Fox, MD, Trauma, Critical Care, Emergency Surgery, Cooper University Hospital, 1 Cooper Plaza, Camden, NJ 08103; email: fox-nicole@cooperhealth.edu.
Introduction
Blunt traumatic aortic injury (BTAI) is the second most common cause of death in trauma patients. Eighty percent of patients with BTAI will die before reaching a trauma center. For patients who survive to hospital arrival, 50% will die within 24 hours. This significant mortality rate is related to the high incidence (40%) of severe associated injuries. The primary mechanism associated with BTAI is motor vehicle crashes (70%); however, BTAI also occurs as a result of motorcycle crashes, falls from height, auto versus pedestrian, and thoracic crush injuries.[1] The issues of how to diagnose, treat, and manage BTAI were first addressed by the Eastern Association for the Surgery of Trauma (EAST) in the practice management guidelines (PMGs) on this topic published in 2000.[2] The literature search for the previous guideline ended in 1997. During the past 15 years, there have been rapid advances in the management of BTAI. As a result, the EAST guidelines committee decided to develop updated guidelines for this topic using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework recently adopted by EAST.[3]
The GRADE framework is used by more than 70 international societies and organizations worldwide. It provides a systematic and transparent framework for clarifying questions, determining outcomes of interest, summarizing evidence, and moving from evidence to recommendations. Within GRADE, evidence is rated across studies for specific clinical outcomes that are important to patients. Recommendation strength and direction are based on evidence quality and the balance between outcomes and patient values and preferences.
There are several issues identified as relevant to this PMG update. Advances in technology and changes in practice occurred since the publication of the last guideline that affected the evaluation and management of BTAI. Areas of focus include the choice of diagnostic radiologic imaging, type of operative repair (open vs. endovascular), and timing of operative repair (immediate vs. delayed).
Objectives
The objective of this guideline was to evaluate the choice of diagnostic imaging (chest computed tomography [CT] with intravenous contrast vs. conventional catheter-based angiography), type of surgical intervention (endovascular vs. open), and timing of surgical intervention (immediate vs. delayed) for patients with BTAI. The Population (P), Intervention (I), Comparator (C) and Outcome (O) questions are defined as follows:
PICO Question 1
In patients with suspected BTAI (P), should CT of the chest with intravenous contrast (I) be used versus conventional catheter-based angiography (C) for the identification of clinically significant BTAI (O)?
PICO Question 2
In patients with BTAI (P), should endovascular (I) repair be performed versus open repair (C) to minimize risk of mortality, stroke, paraplegia, and renal failure (O)?
PICO Question 3
In patients with BTAI (P), should timing of repair be delayed (I) or immediate (C) to minimize risk of mortality, stroke, paraplegia, and renal failure (O)?
Identification of References
With the assistance of an informationist, a search of the National Library of Medicine and the National Institutes of Health MEDLINE database was conducted using (www.pubmed.gov) with citations published between 1998 and 2013. We used the “related articles” function to broaden the search and scan all citations for relevance. In addition to the electronic search, we manually searched the bibliographies of recent reviews and articles. Articles were limited to those in the English language involving human subjects. Letters to the editor, case reports, book chapters, and review articles were excluded. These articles were reviewed by the committee chair, and the final reference list of 60 citations was distributed to the remainder of the study group for review. Of these, 51 articles were felt to be appropriate for the construction of these guidelines and included in the construction of tables of the summary of findings.
Outcome Measure Types
Per the GRADE approach, outcomes were chosen by the committee and rated in importance from 1 to 9, with scores of 7 to 9 representing critical outcomes. For PICO Question 1, the following outcomes were considered by the committee members: identification of clinically significant aortic injury, rapid diagnosis of aortic injury, complications associated with invasive procedures, cost, and patient transport. For PICO Questions 2 and 3, the following outcomes were considered by committee members: mortality, paraplegia, stroke, acute renal failure, length of stay, and cost. Not all of these criteria were deemed “critical” by the committee for the decision-making process within the GRADE framework. Therefore, the critical outcome for PICO Question 1 was determined to be the identification of clinically significant aortic injury. The critical outcomes for PICO Question 2 were mortality, stroke, paraplegia, and renal failure. The critical outcomes for PICO Question 3 were mortality, stroke, paraplegia, and acute renal failure.
Data Extraction and Methodology
PICO Question 1
A systematic review of the MEDLINE database using PubMed was performed with the search terms angiography, blunt aortic injury, blunt thoracic aortic injury, computed chest tomography, and CTA limited to dates from 1998 to 2013. Studies reporting total and false positives as well as total and false negatives for the use of CT with intravenous contrast and aortography were included for further review. Results for the sensitivity and specificity of both diagnostic tests were not pooled because of intrinsic limitations of the study of diagnostic test accuracy in different settings such as increased heterogeneity, nonstandardized designs, quality of testing, and incomplete confirmatory testing (intraoperative findings) in every patient. Six articles contained the necessary information to construct Forest plots for sensitivity and specificity and were deemed appropriate for the construction of the guideline.
PICO Question 2
A similar systematic search of the National Library of Medicine and the National Institutes of Health MEDLINE database was performed using . Search terms included traumatic aortic injury, blunt aortic injury, blunt aortic trauma, endovascular aortic repair, and open aortic repair. Additional references were identified by using two previously published meta-analyses that reported on studies published from 1990 to December 2010.[4][5]Articles were reviewed by the committee chair, and the final reference list of 40 citations was distributed to the remainder of the study group for review. Of these, 38 articles were felt to be appropriate for the construction of these guidelines. One article that reported results on an analysis of a large national administrative database was ultimately excluded because of having a methodology significantly different from the rest of the studies.[6] When comparing open versus endovascular repair, a total of 37 studies reported the outcome of mortality, 21 reported incidence of paralysis, and 12 reported incidence of stroke. With regard to renal failure, the available literature did not provide sufficient or consistent measurements across the studies, specifically if the onset of renal failure occurred before or after surgical intervention. Therefore, this outcome was not able to be included in the meta-analysis.
PICO Question 3
A similar systematic review of the MEDLINE database was performed using search terms blunt aortic injury, traumatic aortic injury repair, immediate repair of blunt thoracic aortic injury, and delayed repair of blunt thoracic aortic injury limited to dates from 1998 to 2013. No randomized trials comparing delayed versus early repair have been performed for BTAI. A final list of seven articles was reviewed by the study group. The outcomes of interest were mortality (reported in all studies), stroke (one study), paraplegia (three studies), and renal failure (three studies).
For PICO Questions 2 and 3, the data for each included article were pooled, and relative risks (RRs) were calculated as measures of effect for dichotomous outcomes using Review Manager (RevMan, Cochrane Collaboration, version 5.2). Potential heterogeneity exists because of population differences as well as different types of surgery performed and how patients are defined. We examined these differences across studies to assess the clinical and methodological heterogeneity. For the meta-analysis, we used RevMan to calculate the Q statistic, and then the I[2] statistic (%) was used to determine the proportion of variation between studies attributable to heterogeneity and categorized as “low” (25–49%), “moderate” (50–74%), or “high” (74–100%). We also used the χ[2] test for heterogeneity and examined the confidence intervals (CIs) for overlap, with decreasing overlap representing increasing heterogeneity. All studies were analyzed using a random-effects model. Tables with summary of findings were constructed using GRADEpro (GRADE Working Group, version 3.2).
Results for PICO Question 1
In patients with suspected BTAI (P), should CT of the chest with intravenous contrast (I) be used versus conventional catheter-based angiography (C) for the identification of clinically significant injury (O)?
Qualitative Synthesis
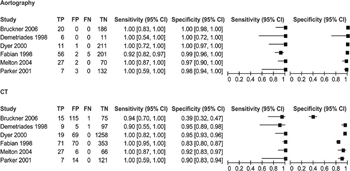
Figure 1: Forest plot of sensitivity and specificity for CTA and angiography.
Conventional angiography was considered the criterion standard for the diagnosis of BTAI for decades. During the past 20 years, however, CT of the chest with intravenous contrast has evolved as a valid screening and diagnostic modality for BTAI because of its availability, rapidity, and ability to diagnose additional intra-thoracic injuries. In the BTAI PMG published by EAST in 2000, the committee made a Level II recommendation stating that “computed tomography of the chest is a useful diagnostic tool for both screening and diagnosis of BTAI. Spiral or helical computed tomographic scanners have an extremely high negative predictive value and may be used alone to rule out BTAI. When these scanners are used, angiography may be reserved for patients with indeterminate scans.”[2] Since the original EAST PMG publication, rapid advances in CT technology occurred with the evolution of helical, spiral, and multigated CT scanners. Nine studies of relevance to this PICO question published after 1997 were identified. Six of these studies contained adequate information to formulate Forest plots of sensitivity and specificity (Fig. 1).
In 1998, Fabian et al.[7] evaluated 494 patients; 71 had a diagnosis of BTAI. Sensitivity and negative predictive value were 100% for CT versus 92% and 97%, respectively, for aortography. Also in 1998, Demetriades et al.[8]identified 9 of 112 trauma patients with BTAI. CT scan identified eight of these injuries. The single injury not identified on CT was a brachiocephalic intimal tear on which the CT images did not include the area of injury. Melton et al.,[9] Parker et al.,[10] and Dyer et al.[11] in separate investigations with a total of 1,802 patients and 64 cases of BTAI found CT sensitivity to be 100%. In 2006, Bruckner et al.[12] analyzed the results of 206 patients who underwent CT followed by aortography. A total of 16 patients had a diagnosis of BTAI; CT failed to identify one injury. The authors noted that this single false-negative scan was performed in 1997 on an older-generation scanner. The aortic injury subsequently identified with aortography was subtle and managed nonoperatively. Overall sensitivity of CT in the study of Bruckner et al. was 95%, and the negative predictive value was 99%.
It is important to note that this PICO question does not address the screening chest x-ray (CXR). This topic was well outlined in the 2000 EAST PMG on BTAI, and the literature on screening CXR has not changed significantly since that time (although we have not conducted an exhaustive systematic review of the topic for this update). Any patient with suspicious findings on CXR or those injured by significant deceleration or acceleration mechanisms should undergo further workup.[2] Since cases of BTAI occur in patients with a normal CXR finding, any clinical suspicion of BTAI should be pursued further regardless of mechanism or CXR findings.
Quantitative Synthesis (Meta-analysis)
Forest plots of sensitivity and specificity were constructed for both CT and aortography. Six studies directly compared CT and aortography. The Forest plots indicate that the sensitivity of both tests is very high. Overall specificity is lower for CT as compared with aortography, indicating that there may be a higher number of “false-positive” results when using CT scan. It must be noted that according to the Cochrane Review Handbook for Systematic Reviews of Diagnostic Test Accuracy, “the statistical aspects of a systematic review of diagnostic test accuracy are more challenging than reviews of interventions.”[13] The RevMan statistical program used for this PMG does not pool sensitivity and specificity, calculate weights for Forest plots, or provide measures of heterogeneity.
Grading the Evidence
With the use of the GRADE framework for evaluating the data specifically related to the outcome of identification of clinically significant injury, no serious risk of bias, inconsistency, indirectness, imprecision, or publication bias was found. Therefore, the overall quality of evidence was low. Per GRADE methodology, if sensitivities and specificities are similar for the diagnostic tests in question, then the preference for one modality over the other may come from the availability of one modality over the other, ease of use, and the value of other secondary information obtained from the diagnostic test.[13]
Recommendation
Within the GRADE framework, once the overall quality of evidence across studies and outcomes is determined, the guideline panel formulates a recommendation that considers the following: quality of evidence, patients’ values and preferences, and cost/resource use. Despite the overall quality of evidence being low, the panel considered that most patients would place a high value on identification of clinically significant BTAI. The sensitivity of CT of the chest is comparable with aortography. There are also a higher number of “false positives” with CT of the chest, indicating that this screening modality may potentially identify minimal aortic injuries not identified on aortography. Furthermore, CT of the chest with intravenous contrast has the advantage of being readily available, less invasive, being less time consuming, and allowing for identification of other intrathoracic injuries. All of these factors resulted in the formulation of a strong recommendation by the committee. Within the GRADE framework, a strong recommendation implies that most individuals would want the recommended course of action, and only a small proportion would not.
In patients with suspected BTAI, we strongly recommend the use of CT scan of the chest with intravenous contrast for diagnosis of clinically significant BTAI.
Results for PICO Question 2
In patients with BTAI (P), should endovascular (I) repair be performed versus open repair (C) to minimize mortality, stroke, paraplegia, and renal failure (O)?
Qualitative Synthesis
The first acute repair of aortic rupture was reported in the 1950s by DeBakey et al. For decades, open repair of aortic injuries was considered the standard of care. In 1997, Kato et al.[14] published the first case report of endovascular stent graft repair of BTAI. Three of 10 patients in this case series had a traumatic aortic aneurysm treated with an endovascular stent. By 2007, there were 284 cases of BTAI treated with endovascular stent grafts from 62 centers reported in the literature. Advances in technology and training paradigms occurred in tandem with the introduction of endovascular stents as a treatment modality for BTAI and resulted in a rapid shift in management of this injury. Early use of stent grafts to repair traumatic aortic injuries was considered “off label.” However, in 2012, the Medtronic Valiant Thoracic Stent Graft with the Captivia Delivery System was approved by the Food and Drug Administration for the treatment of BTAI.[15] To our knowledge, this is the only Food and Drug Administration–approved device for BTAI.
In 2011, the Society for Vascular Surgery (SVS) released clinical practice guidelines for endovascular repair of traumatic thoracic aortic injury. The society proposed a weak recommendation in favor of endovascular repair. The SVS used GRADE to develop its recommendations. The review included 7,768 patients from 139 studies (1990–2009), but the authors did not limit eligibility criteria based on study design.[16] Therefore, 112 studies included were case series (noncomparative), and 27 were comparative observational studies. The overall quality of evidence was determined to be “very low.” The group placed the highest value on the same outcomes chosen for this PMG: mortality, stroke, and paraplegia. Overall mortality was lower for patients who underwent endovascular repair versus open repair (9% vs. 19%). The risk of paraplegia was also lower for endovascular repair as compared with open (3% vs. 9%), and there were no differences in the incidence of stroke between the two groups.
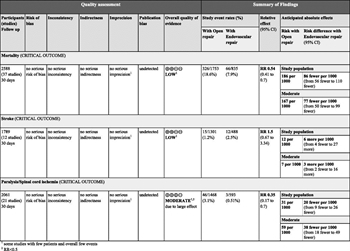
Figure 3: GRADE profile for endovascular versus open repair of BTAI.
To our knowledge, there are no randomized studies comparing open (OR) versus endovascular (TEVAR) repair of blunt thoracic aortic injury. For the purposes of this guideline, studies that reported results on only one kind of approach (TEVAR or OR) were excluded, and only articles with comparative reports of both approaches were included in the final analysis. Forty-five comparative studies (1997–2013) were identified, and 37 were ultimately included in the construction of the evidence profile (Fig. 3).[17–52] Mortality data were available in all of the identified studies, and overall mortality was lower for endovascular as compared with open repair (8% vs. 19%). Rates of paraplegia were available in 12 of the 37 studies, and the incidence of paraplegia was also lower for endovascular versus open repair (0.5% vs. 3%). Rates of stroke were available in 21 of 37 studies, and the incidence of stroke was slightly higher in the endovascular group as compared with open (2.5% vs. 1%).
Endovascular repair is now performed more commonly than open repair in patients with BTAI. The 2008 study by American Association for the Surgery of Trauma (AAST) demonstrated that 65% of the patients were treated with endovascular stent grafts in 2007 as compared with 0% in 1997.[28] Experience and training in endovascular repair have steadily increased, with a resultant decrease in exposure to open repair. One of the primary concerns with endovascular repair in earlier studies was the reported high rate of device-related complications. In the AAST series, 32 device-related complications developed in 25 patients (20%). Although the most common complication was endoleak (14%), other complications included access-vessel injuries, occlusion of the left subclavian or left common carotid artery, and late migration and thrombosis of the stent graft.[28] During the past 5 years, however, reports demonstrate that the rate of device-related complications has decreased significantly. In 2014, Azizzadeh et al.[52] published the follow-up results of 82 consecutive patients who underwent endovascular repair for BTAI. Average time to follow-up was 2.3 years, and the incidence of device-related complications was 2.4%. All patients should be evaluated preoperatively for the appropriateness of endovascular repair because there are contraindications to endovascular repair. These include aortic diameter less than 15 mm, involvement of tear into midarch requiring coverage of the left common carotid artery, and left vertebral origin on the aortic arch with an uncollateralized posterior inferior communicating artery. It is also important to note that if TEVAR is used, the treating center must have the ability to convert to open repair if necessary.
Long-term follow-up of patients who have endovascular stent grafts placed is required to monitor longevity and status of the graft. There was insufficient literature to make an evidence-based recommendation on the frequency of follow-up. In their 2011 guidelines, the SVS did not provide a recommendation for long-term evaluation and stated that the follow-up of patients after TEVAR “remains in evolution.” The RESCUE trial, published in 2013, defined its follow-up protocol as follows: “a CTA or magnetic resonance angiogram at 1, 6, and 12 months and annually thereafter for 5 years. Multiple view chest x-rays will also be acquired at 1, 3, and 5 years to assess for device integrity.”[53] This remains an area in which further research is necessary.
Quantitative Synthesis (Meta-analysis)
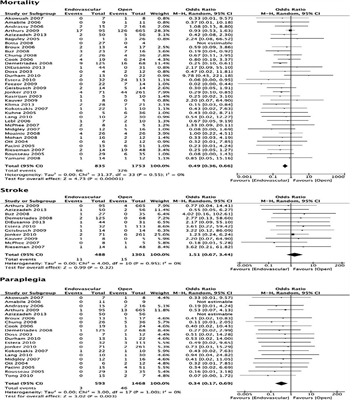
Figure 2: Forest plots for endovascular versus open repair of BTAI.
Thirty-seven studies were included in the meta-analysis. Endovascular repair was associated with reduced mortality rates, with an RR of 0.56 (95% CI, 0.44–0.73). Of note, the I[2] statistic was 0%, falling into the “low” heterogeneity category, indicating that the studies are comparable. Endovascular repair was associated with comparable stroke rates, with an RR of 1.48 (95% CI, 0.67–3.27). The I[2] was 0%, indicating that the studies are comparable. Endovascular repair was associated with significantly reduced paraplegia rates, with an RR of 0.36 (95% CI, 0.19–0.71). The I[2] was 0%, indicating that the studies are comparable (Fig. 2).
Grading the Evidence
With the use of the GRADE framework for evaluating the data specifically related to the outcome of mortality, no serious risk of bias, inconsistency, indirectness, imprecision, or publication bias was found. The evidence could not be upgraded, and therefore, the overall quality was low. For the outcome of stroke, no serious risk of bias, inconsistency, indirectness, imprecision, or publication bias was found, and the evidence could not be upgraded. The overall quality of evidence for this outcome was low. For the outcome of paraplegia, no serious risk of bias, inconsistency, indirectness, imprecision, or publication bias was found. The evidence for this outcome was upgraded for a strong association, and the overall quality of evidence was moderate. An evidence profile was constructed using the Gradepro software by importing the data from RevMan (Fig. 3).
Recommendation
Despite the overall quality of evidence being low (mortality, stroke) to moderate (paraplegia), the panel considered that most patients would place a high value on a less invasive procedure that carries a significantly lower risk of blood loss, mortality, and paraplegia and a comparable risk of stroke. The panel also considered the fact that endovascular repair is performed more frequently than open repair, resulting in decreased experience with and training in open repair. In addition, initial concerns regarding a high rate of device-related complications seem unfounded as the current literature suggests that complication rates are low and continue to improve as technology evolves. All of these factors resulted in the formulation of a strong recommendation by the committee. Within the GRADE framework, a strong recommendation implies that most individuals would want the recommended course of action, and only a small proportion would not.
In patients diagnosed with BTAI, we strongly recommend the use of endovascular repair in patients who do not have contraindications to endovascular repair.
Results for PICO Question 3
In patients with BTAI (P), should timing of repair be delayed (I) or immediate (C) to minimize mortality, stroke, paraplegia, and renal failure (O)?
Qualitative Synthesis
The risk of rupture of contained BTAI is highest within the first 24 hours of injury. For this reason, immediate repair of BTAI was advocated and considered the standard of care for decades. In the 2000 PMG, a Level II recommendation was made for prompt repair unless patients “have more immediately life threatening injuries that require intervention such as emergent laparotomy or craniotomy, or if the patient is a poor operative candidate due to age or co-morbidities.”[2]
For the purposes of this PMG, seven comparative studies (1997–2013) were identified, and all were included in the construction of the evidence profile. The 2004 study by Hemmila et al.[54] reported data on two separate subsets of patients, a population from their own institution and a population from the National Trauma Data Bank. Mortality data were available in all studies, and overall mortality was lower for delayed repair versus immediate repair (9% vs. 21%). Rates of paraplegia were reported in four studies and were significantly lower for delayed repair as compared with immediate repair (0.6% vs. 5.5%). Incidence of stroke was evaluated in one study and was lower in the delayed group (7% vs. 9%). There was no difference in the incidence of renal failure (reported in three studies) between the two groups (9.3% delayed vs. 8.6% immediate)
In 1998, Fabian et al.[7] prospectively evaluated the use of antihypertensive therapy in patients with BTAI. Of 71 patients with BTAI, 75% received a regimen of β-blockers with or without nitroprusside. They found that no in-hospital ruptures occurred in the delayed management or nonoperative management groups. The use of an antihypertensive regimen decreases aortic wall stress and tension and significantly reduces the risk of aortic rupture. Before this investigation, it was demonstrated that 12% of patients with BTAI sustained in-hospital aortic rupture. The success of anti-hypertensive regimens in preventing rupture has resulted in the practice of delayed repair of BTAI in both high- and low-risk patients. Any patient with BTAI should be immediately started and maintained on an antihypertensive regimen to prevent aortic rupture. These regimens are used to maintain the systolic blood pressure within a “normal” range, generally less than 120 mm Hg.
Delayed repair of BTAI was traditionally reserved for high-risk patients with major associated injuries or severe comorbidities. However, following publication of the 1998 article by Fabian et al., the use of antihypertensive regimens became widespread, and delayed repair was extended to low-risk trauma patients. In the 2009 AAST prospective study, 35% of patients overall underwent delayed repair.[55] This was also the only one of the six comparative studies used to construct this PMG, which stratified patients in the early and delayed repair groups by the presence/absence of major extrathoracic injuries.[56][57] The benefits of delayed repair in terms of mortality (22% early vs. 3% delayed), paraplegia (3% early vs. 0% delayed), and renal failure (38% early vs. 29% delayed) for patients with major extrathoracic injuries are significant. Incidence of stroke was not evaluated in this study. The mortality benefit of delayed repair (14% early vs. 8% delayed) was still present in patients without major extrathoracic injuries, although not as significant. In patients without major extrathoracic injuries, rates of paraplegia (1% early vs. 3% delayed) and renal failure (6% early vs. 8% delayed) were higher in the group that underwent delayed repair. The authors partially attributed the higher complication rate in the early repair group without major extrathoracic injuries to a higher rate of early deaths in this group. In light of the clear survival benefit associated with delayed repair, the authors advocated delayed repair in “all patients irrespective of risk factors.”
Patient at a high risk of aortic rupture, based on clinical suspicion, imaging characteristics, and/or grade of injury should not be considered for delayed repair. This would include patients with Grade 3 and 4 injuries, which are defined as BTAI with pseudoaneurysm (Grade 3) and BTAI with active extravasation (Grade 4). In addition, clinical or radiographic evidence of pseudocoarctation may be an indication for urgent repair.
It is important to note that the role of medical management alone (without surgical or endovascular treatment) for “minor” BTAI was discussed and considered by the committee. Although this is an important issue, at this time, there is insufficient evidence to formulate a recommendation on this topic. It remains an area for further research.
Quantitative Synthesis (Meta-analysis)

Figure 4: Forest plots for delayed versus open repair of BTAI.
Seven studies were included in the meta-analysis. Delayed repair was associated with reduced mortality rates, with an RR of 2.07 (95% CI, 1.03–4.15). Of note, the I[2] statistic was 51%, falling into the “moderate” heterogeneity category. The risk of stroke with delayed repair was lower but was evaluated in only one study, with an RR of 1.36 (95% CI, 0.29–6.34). Delayed repair was associated with significantly reduced paraplegia rates (evaluated in four studies), with an RR of 5.90 (95% CI, 1.51–22.97). The I[2] was 12%, falling into the “low” heterogeneity category, indicating that the studies are comparable. Rates of renal failure were comparable (evaluated in three studies), with an RR of 0.87 (CI 0.42–1.79). The I[2] was 59%, falling into the “moderate” heterogeneity category (Fig. 4).
Grading the Evidence
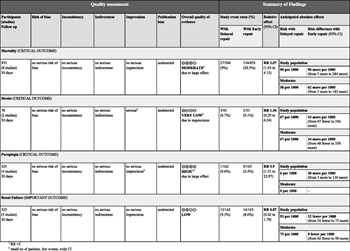
Figure 5: GRADE profile for delayed versus early repair of BTAI.
With the use of the GRADE framework for evaluating the data specifically related to the outcome of mortality, no serious risk of bias, inconsistency, indirectness, imprecision, or publication bias was found. The evidence was upgraded for a strong association, resulting in the overall quality of evidence being moderate. For the outcome of stroke, no serious risk of bias, inconsistency, indirectness, or publication bias was found. However, the evidence was downgraded for imprecision, and the overall quality was very low. For the outcome of paraplegia, no serious risk of bias, inconsistency, indirectness, imprecision, or publication bias was found. The evidence was upgraded for a very strong association, and the overall quality was high. For the outcome of renal failure, no serious risk of bias, inconsistency, indirectness, imprecision, or publication bias was found. Therefore, the overall quality of evidence was low. An evidence profile was constructed using the Gradepro software by importing the data from RevMan (Fig. 5).
Recommendation
The overall quality of evidence ranged from very low (stroke) to high (paraplegia). However, the panel considered that most patients would place a high value on BTAI repaired in a delayed fashion because it results in decreased mortality and paraplegia. Rates of renal failure were nearly identical. The panel discussed the fact that the patients who benefit the most from delayed repair are those who have major associated injuries. These patients clearly require resuscitation and treatment of immediately life-threatening injuries before aortic repair. The data are not as clear for patients without associated injuries who have no reason to undergo delayed repair. The panel does notadvocate delaying repair of BTAI (e.g., until the following weekday morning) merely for surgeon convenience. Although the studies included in the evidence profile demonstrated decreased incidence of mortality, stroke, and paraplegia with delayed repair, it should be noted that the reason the majority of patients in these studies underwent delayed repair was because they had associated life-threatening injuries and/or a requirement for further resuscitation. Only one study evaluated the effect of delayed repair in a select group of patients without major associated injuries, and the number of patients in this group was small (n = 108). It is important to consider that in that group of patients, the benefit of delayed repair was only related to mortality. The incidence of paraplegia and renal failure in this subset of patients was higher. The consideration of these factors resulted in the formulation of a conditional recommendation by the committee. Within the GRADE framework, a conditional recommendation implies that the majority of individuals would want the recommended course of action but many would not.
In patients diagnosed with BTAI, we suggest delayed repair. It is critical that effective blood pressure control with antihypertensive medication is used in these patients.
Using These Guidelines in Clinical Practice
These guidelines represent a detailed summary and comprehensive overview of the literature regarding the evaluation and treatment of BTAI. They are meant to inform the decision-making process and not replace clinical judgment. Patients with BTAI have a high mortality rate. The literature available for review strongly supports the use of CT of the chest with intravenous contrast for the identification of clinically significant injury and the use of endovascular repair of BTAI in patients without contraindications. The literature available for review supports delayed repair of BTAI in patients with the caveat that effective antihypertensive regimens must be used between the time of diagnosis and definitive repair.
Conclusion
In summary, we propose three important and evidence-based recommendations regarding BTAI, which were formulated using the GRADE methodology. First, we strongly recommend CT of the chest with intravenous contrast for the identification of clinically significant BTAI. Second, we strongly recommend the use of endovascular repair in patients with BTAI who do not have contraindications to endovascular repair. Finally, we suggest the use of delayed repair in patients with BTAI and emphasize that effective blood pressure control with antihypertensive medication must be used in these cases.
Authorship
N.F. and E.R.H. conceived the study.
N.F. created the PICO questions. T.M.S. and T.C.F. assisted with finalizing the PICO questions. All listed authors with the exception of Ms. Seal and P.D. voted regarding the outcomes of interest for these PICO questions.
N.F. and D.S. performed the entire literature search, read all of the abstracts, and selected the articles for review. N.F., D.S., E.R.H., J.H.B., S.C.B., J.J.C., K.H., D.R.K., A.A.M., M.L.M., K.N., L.B.P. and R.T. reviewed and summarized the selected articles.
N.F. and J.H.S. extracted the data from the selected articles. J.H.S. and N.F. entered the extracted data into the RevMan and GRADEpro programs and evaluated the results for recommendations. P.D. assisted with data analysis and the RevMan and GRADEpro software programs.
N.F., D.S. and E.R.H. wrote the manuscript. T.M.S. and T.C.F. contributed equally to this work as senior mentors and should be noted as cosenior authors. E.R.H. reviewed this article for methodological content and made critical revisions to the final draft. All authors participated in the critical review of all versions of this article.
Acknowledgements
We thank the Eastern Association for the Surgery of Trauma and the EAST foundation for the opportunity to write this article and the detail-oriented peer review of this article by the guidelines committee.
Disclosure
The authors declare no conflicts of interest.
References
- Demetriades D. Blunt thoracic aortic injuries: crossing the rubicon. J Am Coll Surg 2011; 214 (3): 247–259.
- Nagy K, Fabian T, Rodman G, Fulda G, Rodriguez A, Mirvis S. Guidelines for the diagnosis and management of blunt aortic injury: an EAST Practice Management Guidelines Work Group. J Trauma 2000; 48 (6): 1128–1143.
- Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, Dahm P; Eastern Association for the Surgery of Trauma Practice Management Guidelines Ad Hoc Committee. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg 2012; 73 (5 Suppl 4): S283–S287.
- Murad MH, Rizvi AZ, Malgor R, Carey J, Alkatib AA, Erwin PJ, Lee WA, Fairman RM. Comparative effectiveness of the treatments for thoracic aortic transection. J Vasc Surg 2011; 53 (1): 193–199.
- Karmy-Jones R, Ferrigno L, Teso D, Long WB 3rd, Shackford S. Endovascular repair compared with operative repair of traumatic rupture of the thoracic aorta: a nonsystematic review and a plea for trauma-specific reporting guidelines. J Trauma 2011; 71 (4): 1059–1072.
- Hong MS, Feezor RJ, Lee WA, Nelson PR. The advent of thoracic endovascular aortic repair is associated with broadened treatment eligibility and decreased overall mortality in traumatic thoracic aortic injury. J Vasc Surg 2011; 53 (1): 36–42.
- Fabian TC, Davis KA, Gavant ML, Croce MA, Melton SM, Patton JH, Haan CK, Weiman DS, Pate JW. Prospective study of blunt aortic injury: helical CT is diagnostic and antihypertensive therapy reduces rupture. Ann Surg 1998; 227 (5): 666–676.
- Demetriades D, Gomez H, Velmahos GC, Asensio JA, Murray J, Cornwell EE, Alo K, Berne TV. Routine helical computed tomographic evaluation of the mediastinum in high-risk blunt trauma patients. Arch Surg 1998; 13 (10): 1084–1088.
- Melton SM, Kerby JD, McGiffin D, McGwin G, Smith JK, Oser RF, Cross JM, Windham ST, Moran SG, Hsia J, et al. The evolution of chest computed tomography for the definitive diagnosis of blunt aortic injury: a single-center experience. J Trauma. 2004; 56 (2): 243–250.
- Parker MS, Matheson TL, Rao AV, Sherbourne CD, Jordan KG, Landay MJ, Miller GL, Summa JA. Making the transition: the role of helical CT in the evaluation of potentially acute thoracic aortic injuries. AJR Am J Roentgenol. 2001; 176 (5): 1267–1272.
- Dyer DS, Moore EE, Ilke DN, McIntyre RC, Bernstein SM, Durham JD, Mestek MF, Heinig MJ, Russ PD, Symonds DL, et al. Thoracic aortic injury: how predictive is mechanism and is chest computed tomography a reliable screening tool? A prospective study of 1,561 patients. J Trauma. 2000; 48 (4): 673–682.
- Bruckner BA, DiBardino DJ, Cumbie TC, Trinh C, Blackmon SH, Fisher RG, Mattox KL, Wall MJ. Critical evaluation of chest computed tomography scans for blunt descending thoracic aortic injury. Ann Thorac Surg. 2006; 81 (4): 1339–1346.
- Diagnostic Test Accuracy Working Group. Handbook for DTA Reviews. http://srdta.cochrane.org/handbook-dta-reviews. Accessed May 15, 2014.
- Kato N, Hirano T, Ishida M, Shimono T, Cheng SH, Yada I, Takeda K. Acute and contained rupture of the descending thoracic aorta: treatment with endovascular stent grafts. J Vasc Surg. 2003; 37 (1): 100–105.
- US Food and Drug Administration. Medical Devices. Medtronic Valiant Thoracic Stent Graft with the Captivia Delivery System—P100040/S008. Available at: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm327486.htm. Accessed June 17, 2014.
- Lee WA, Matsumura JS, Mitchell RS, Farber MA, Greenberg RK, Azizzadeh A, Murad MH, Fairman RM. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011; 53 (1): 187–192.
- Akowuah E, Baumbach A, Wilde P, Angelini G, Bryan AJ. Emergency repair of traumatic aortic rupture: endovascular versus conventional open repair. J Thorac Cardiovasc Surg. 2007; 134 (4): 897–901.
- Amabile P, Rollet G, Vidal V, Collart F, Bartoli JM, Piquet P. Emergency treatment of acute rupture of the descending thoracic aorta using endovascular stent-grafts. Ann Vasc Surg. 2006; 20 (6): 723–730.
- Andrassy J, Weidenhagen R, Meimarakis G, Lauterjung L, Jauch KW, Kopp R. Stent versus open surgery for acute and chronic traumatic injury of the thoracic aorta: a single-center experience. J Trauma. 2006; 60 (4): 765–771.
- Arthurs ZM, Starnes BW, Sohn VY, Singh N, Martin MJ, Andersen CA. Functional and survival outcomes in traumatic blunt thoracic aortic injuries: an analysis of the National Trauma Databank. J Vasc Surg. 2009; 49 (4): 988–994.
- Azizzadeh A, Charlton-Ouw KM, Chen Z, Rahbar MH, Estrera AL, Amer H, Coogan SM, Safi HJ. An outcome analysis of endovascular versus open repair of blunt traumatic aortic injuries. J Vasc Surg. 2013; 57 (1): 108–114.
- Baguley CJ, Sibal AK, Alison PM. Repair of injuries to the thoracic aorta and great vessels: Auckland, New Zealand 1995–2004. ANZ J Surg. 2005; 75 (6): 383–387.
- Botta L, Russo V, Savini C, Buttazzi K, Pacini D, Lovato L, La Palombara C, Parlapiano M, Di Bartolomeo R, Fattori R. Endovascular treatment for acute traumatic transection of the descending aorta: focus on operative timing and left subclavian artery management. J Thorac Cardiovasc Surg. 2008; 136 (6): 1558–1563.
- Broux C, Thony F, Chavanon O, Bach V, Hacini R, Sengel C, Blin D, Lavagne P, Girardet P, Jacquot C. Emergency endovascular stent graft repair for acute blunt thoracic aortic injury: a retrospective case control study.Intensive Care Med. 2006; 32 (5): 770–774.
- Buz S, Zipfel B, Mulahasanovic S, Pasic M, Weng Y, Hetzer R. Conventional surgical repair and endovascular treatment of acute traumatic aortic rupture. Eur J Cardiothorac Surg. 2008; 33 (2): 143–149.
- Chung J, Owen R, Turnbull R, Chyczij H, Winkelaar G, Gibney N. Endovascular repair in traumatic thoracic aortic injuries: comparison with open surgical repair. J Vasc Interv Radiol. 2008; 19 (4): 479–486.
- Cook J, Salerno C, Krishnadasan B, Nicholls S, Meissner M, Karmy-Jones R. The effect of changing presentation and management on the outcome of blunt rupture of the thoracic aorta. J Thorac Cardiovasc Surg. 2006; 131 (3): 594–600.
- Demetriades D, Velmahos GC, Scalea TM, Jurkovich GJ, Karmy-Jones R, Teixeira PG, Hemmila MR, O’Connor JV, McKenney MO, Moore FO. Operative repair or endovascular stent graft in blunt traumatic thoracic aortic injuries: results of an American Association for the Surgery of Trauma Multicenter Study. J Trauma. 2008; 64 (3): 561–570.
- Di Eusanio M, Folesani G, Berretta P, Petridis FD, Pantaleo A, Russo V, Lovato L, Di Bartolomeo R. Delayed management of blunt traumatic aortic injury: open surgical versus endovascular repair. Ann Thorac Surg. 2013; 95 (5): 1591–1597.
- Doss M, Balzer J, Martens S, Wood JP, Wimmer-Greinecker G, Fieguth HG, Moritz A. Surgical versus endovascular treatment of acute thoracic aortic rupture: a single-center experience. Ann Thorac Surg. 2003; 76 (5): 1465–1469.
- Durham CA, McNally MM, Parker FM, Bogey WM, Powell CS, Goettler CE, Rotondo MF, Stoner MC. A contemporary rural trauma center experience in blunt traumatic aortic injury. J Vasc Surg. 2010; 52 (4): 884–889.
- Feezor RJ, Hess PJ Jr, Martin TD, Klodell CT, Beaver TM, Lottenberg L, Martin LC, Lee WA. Endovascular treatment of traumatic thoracic aortic injuries. J Am Coll Surg. 2009; 208 (4): 510–516.
- Geisbüsch P, Leszczynsky M, Kotelis D, Hyhlik-Dürr A, Weber TF, Böckler D. Open versus endovascular repair of acute aortic transections—a non-randomized single-center analysis. Langenbecks Arch Surg. 2009; 394 (6): 1101–1107.
- Jonker FH, Giacovelli JK, Muhs BE, Sosa JA, Indes JE. Trends and outcomes of endovascular and open treatment for traumatic thoracic aortic injury. J Vasc Surg. 2010; 51 (3): 565–571.
- Kasirajan K, Heffernan D, Langsfeld M. Acute thoracic aortic trauma: a comparison of endoluminal stent grafts with open repair and nonoperative management. Ann Vasc Surg. 2003; 17 (6): 589–595.
- Kauvar DS, White JM, Johnson CA, Jones WT, Rasmussen TE, Clouse WD. Endovascular versus open management of blunt traumatic aortic disruption at two military trauma centers: comparison of in-hospital variables. Mil Med. 2009; 174 (8): 869–873.
- Klima DA, Hanna EM, Christmas AB, Huynh TT, Etson KE, Fair BA, Green JM, Madjarov J, Sing RF. Endovascular graft repair for blunt traumatic disruption of the thoracic aorta: experience at a non-university hospital. Am Surg. 2013; 79 (6): 594–600.
- Kokotsakis J, Kaskarelis I, Misthos P, Athanasiou T, Kanakakis K, Athanasiou C, Romana C, Skouteli E, Lioulias A. Endovascular versus open repair for blunt thoracic aortic injury: short-term results. Ann Thorac Surg. 2007; 84 (6): 1965–1970.
- Eggebrecht H, Schmermund A, Herold U, Baumgart D, Martini S, Kuhnt O, Lind AY, Kühne C, Kühl H, Kienbaum P, et al. Endovascular stent-graft placement for acute and contained rupture of the descending thoracic aorta.Catheter Cardiovasc Interv. 2005; 66 (4): 474–482.
- Lang JL, Minei JP, Modrall JG, Clagett GP, Valentine RJ. The limitations of thoracic endovascular aortic repair in altering the natural history of blunt aortic injury. J Vasc Surg. 2010; 52 (2): 290–297.
- Lebl DR, Dicker RA, Spain DA, Brundage SI. Dramatic shift in the primary management of traumatic thoracic aortic rupture. Arch Surg. 2006; 141 (2): 177–180.
- McPhee JT, Asham EH, Rohrer MJ, Singh MJ, Wong G, Vorhies RW, Nelson PR, Cutler BS. The midterm results of stent graft treatment of thoracic aortic injuries. J Surg Res. 2007; 138 (2): 181–188.
- Midgley PI, Mackenzie KS, Corriveau MM, Obrand DI, Abraham CZ, Fata P, Steinmetz OK. Blunt thoracic aortic injury: a single institution comparison of open and endovascular management. J Vasc Surg. 2007; 46 (4): 662–668.
- Moainie SL, Neschis DG, Gammie JS, Brown JM, Poston RS, Scalea TM, Griffith BP. Endovascular stenting for traumatic aortic injury: an emerging new standard of care. Ann Thorac Surg. 2008; 85 (5): 1625–1629.
- Mohan IV, Hitos K, White GH, Harris JP, Stephen MS, May J, Swinnen J, Fletcher JP. Improved outcomes with endovascular stent grafts for thoracic aorta transections. Eur J Vasc Endovasc Surg. 2008; 36 (2): 152–157.
- Ott MC, Stewart TC, Lawlor DK, Gray DK, Forbes TL. Management of blunt thoracic aortic injuries: endovascular stents versus open repair. J Trauma. 2004; 56 (3): 565–570.
- Pacini D, Angeli E, Fattori R, Lovato L, Rocchi G, Di Marco L, Bergonzini M, Grillone G, Di Bartolomeo R. Traumatic rupture of the thoracic aorta: ten years of delayed management. J Thorac Cardiovasc Surg. 2005; 129 (4): 880–884.
- Riesenman PJ, Farber MA, Rich PB, Sheridan BC, Mendes RR, Marston WA, Keagy BA. Outcomes of surgical and endovascular treatment of acute traumatic thoracic aortic injury. J Vasc Surg. 2007; 46 (5): 934–940.
- Rousseau H, Dambrin C, Marcheix B, Richeux L, Mazerolles M, Cron C, Watkinson A, Mugniot A, Soula P, Chabbert V, et al. Acute traumatic aortic rupture: a comparison of surgical and stent-graft repair. J Thorac Cardiovasc Surg. 2005; 129 (5): 1050–1055.
- Tong MZ, Koka P, Forbes TL. Economic evaluation of open vs endovascular repair of blunt traumatic thoracic aortic injuries. J Vasc Surg. 2010; 52 (1): 31–38.
- Yamane BH, Tefera G, Hoch JR, Turnipseed WD, Acher CW. Blunt thoracic aortic injury: open or stent graft repair? Surgery. 2008; 144 (4): 575–580.
- Azizzadeh A, Ray HM, Dubose JJ, Charlton-Ouw KM, Miller CC, Coogan SM, Safi HJ. Outcomes of endovascular repair for patients with blunt traumatic aortic injury. J Trauma Acute Care Surg. 2014; 76: 510–516.
- Khoynezhad A, Azizzadeh A, Donayre C, Matsumoto A, Velazquez O, White R. Results of a multicenter, prospective trial of thoracic endovascular aortic repair for blunt thoracic aortic injury (RESCUE trial). J Vasc Surg. 2013; 57: 899–905.
- Hemmila MR, Arbabi S, Rowe SA, Brandt MM, Wang SC, Taheri PA, Wahl WL. Delayed repair for blunt thoracic aortic injury: is it really equivalent to early repair? J Trauma. 2004; 56 (1): 13–23.
- Demetriades D, Velmahos GC, Scalea TM, Jurkovich GJ, Karmy-Jones R, Teixeira PG, Hemmila MR, O’Connor JV, McKenney MO, Moore FO. Blunt traumatic thoracic aortic injuries: early or delayed repair—results of an American Association for the Surgery of Trauma prospective study. J Trauma. 2009; 66 (4): 967–973.
- Estrera AL, Gochnour DC, Azizzadeh A, Miller CC, Coogan S, Charlton-Ouw K, Holcomb JB, Safi HJ. Progress in the treatment of blunt thoracic aortic injury: 12-year single-institution experience. Ann Thorac Surg. 2010; 90 (1): 64–71.
- Symbas PN, Sherman AJ, Silver JM, Symbas JD, Lackey JJ. Traumatic rupture of the aorta: immediate or delayed repair? Ann Surg. 2002; 235 (6): 796–802.