Blunt Cerebrovascular Injury, Evaluation and Management of
Published 2020
Citation: J Trauma. 88(6):875-887, June 2020
Visual PMG
Authors
Kim, Dennis Y. MD; Biffl, Walter MD; Bokhari, Faran MD; Brakenridge, Scott MD; Chao, Edward MD; Claridge, Jeffrey A. MD, MS; Fraser, Douglas MD; Jawa, Randeep MD; Kasotakis, George MD, MPH; Kerwin, Andy MD; Khan, Uzer MD; Kurek, Stan MD; Plurad, David MD; Robinson, Bryce R.H. MD, MS; Stassen, Nicole MD; Tesoriero, Ron MD; Yorkgitis, Brian DO; Como, John J. MD, MPH
Author Information
From the Division of Trauma/Acute Care Surgery/Surgical Critical Care, Department of Surgery (D.Y.K.), Harbor-UCLA Medical Center, David Geffen School of Medicine at UCLA, Torrance; Trauma Surgery Department, Scripps Memorial Hospital La Jolla (W.B.), La Jolla, California; Department of Trauma and Burn Surgery, Stroger Hospital of Cook County (F.B.), Rush University, Chicago, Illinois; Department of Surgery (S.B.), University of Florida, Gainesville, Florida; Department of Surgery, Jacobi Medical Center (E.C.), Bronx, New York; Department of Surgery, MetroHealth Medical Center (J.A.C., J.J.C.), Cleveland, Ohio; Department of Surgery, UNLV School of Medicine (D.F.), Las Vegas, Nevada; Division of Trauma, Emergency Surgery, and Surgical Critical Care, School of Medicine (R.J.), Stony Brook University, Stony Brook, New York; Department of Surgery, University of Florida College of Medicine — Jacksonville (A.K., B.Y.), Jacksonville, Florida; Department of Surgery, Duke University (G.K.), Durham, North Carolina; Department of Surgery, Western Virginia University (U.K.), Morgantown, West Virginia; Department of Surgery (S.K.), Chippenham-Johnston Willis Medical Center, NorthStar Trauma Surgery, Richmond, Virginia; Department of Surgery, Riverside Community Hospital (D.P.), Riverside, California; Division of Trauma and Critical Care, Department of Surgery, Harborview Medical Center (B.R.H.R.), University of Washington, Seattle, Washington; Division of Acute Care and Trauma Surgery, Department of Surgery, Rochester University Medical Center (N.S.), Rochester, New York; and Department of Surgery, University of Maryland Medical Center (R.T.), Baltimore, Maryland.
Submitted: January 29, 2020, Revised: February 27, 2020, Accepted: March 3, 2020, Published online: March 14, 2020.
This study was presented at the Eastern Association for the Surgery of Trauma Annual Scientific Meeting, 2018, in Lake Buena Vista, Florida on January 12, 2018.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
Address for reprints: Dennis Yong Kim, MD, MMEd, FRCSC, FACS, FCCP, FCCM, Division of Trauma/Acute Care Surgery/Surgical Critical Care, Department of Surgery, Harbor-UCLA Medical Center, David Geffen School of Medicine at UCLA, 1000 West Carson St, Box 42, Torrance, CA 90509; email: dekim@dhs.lacounty.gov.
Abstract
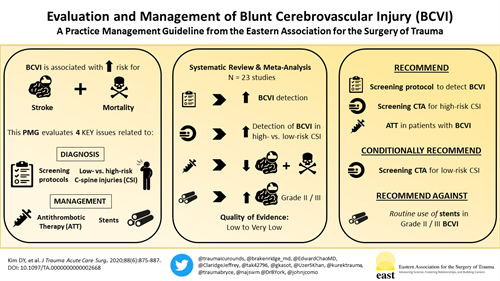
BACKGROUND
Blunt cerebrovascular injuries (BCVIs) are associated with significant morbidity and mortality. This guideline evaluates several aspects of BCVI diagnosis and management including the role of screening protocols, criteria for screening cervical spine injuries, and the use of antithrombotic therapy (ATT) and endovascular stents.
METHODS
Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, a taskforce of the Practice Management Guidelines Committee of the Eastern Association for the Surgery of Trauma performed a systematic review and meta-analysis of currently available evidence. Four population, intervention, comparison, and outcome questions were developed to address diagnostic and therapeutic issues relevant to BCVI.
RESULTS
A total of 98 articles were identified. Of these, 23 articles were selected to construct the guidelines. In these studies, the detection of BCVI increased with the use of a screening protocol versus no screening protocol (odds ratio [OR], 4.74; 95% confidence interval [CI], 1.76–12.78; p = 0.002), as well as among patients with high-risk versus low-risk cervical spine injuries (OR, 12.7; 95% CI, 6.24–25.62; p = 0.003). The use of ATT versus no ATT resulted in a decreased risk of stroke (OR, 0.20; 95% CI, 0.06–0.65; p < 0.0001) and mortality (OR, 0.17; 95% CI, 0.08–0.34; p < 0.0001). There was no significant difference in the risk of stroke among patients with Grade II or III injuries who underwent stenting as an adjunct to ATT versus ATT alone (OR, 1.63; 95% CI, 0.2–12.14; p = 0.63).
CONCLUSION
We recommend using a screening protocol to detect BCVI in blunt polytrauma patients. Among patients with high-risk cervical spine injuries, we recommend screening computed tomography angiography to detect BCVI. For patients with low-risk risk cervical injuries, we conditionally recommend performing a computed tomography angiography to detect BCVI. We recommend the use of ATT in patients diagnosed with BCVI. Finally, we recommend against the routine use of endovascular stents as an adjunct to ATT in patients with Grade II or III BCVIs.
LEVEL OF EVIDENCE
Guidelines, Level III.
Blunt cerebrovascular injuries (BCVIs) are rare yet potentially devastating injuries affecting 1% to 3% of blunt trauma patients.[1–5] Over the past three decades, significant advances have been made in our understanding of the mechanisms and pathophysiology underlying these injuries.[6–9] The development of screening criteria (Table 1)[5][8] and an injury grading scale (Table 2)[11] has been central to our ability to detect, accurately describe, prognosticate, and treat BCVIs.[10][12][13]
Early diagnosis and treatment are critical to minimize the morbidity and mortality associated with BCVIs.[2][4][14–23] One of the most feared complications is a stroke, which may occur in up to 20% of patients, most commonly in the early (<72 hours) postinjury period.[24][25] Early initiation of antithrombotic therapy (ATT) has been demonstrated to decrease the risk for stroke, stroke-related morbidity and mortality, and overall mortality in patients with BCVIs.[1][17][24][26–32]
Since the original Eastern Association for the Surgery of Trauma practice management guideline (PMG) was published in 2010,[33] new data and controversy have emerged regarding various aspects in the diagnosis and management of BCVIs. The following PMG addresses several issues related to the use of screening protocols, screening of cervical spine injuries, the role of ATT, and endovascular stents in patients with BCVI.
Objectives
Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology,[26][34] we defined four population (P), intervention (I), comparator (C), and outcome (O) (PICO) questions:
- PICO question 1: In adult patients with blunt polytrauma (P), should a screening protocol (I) versus no screening protocol (C) be used to detect BCVI (O)?
- PICO question 2.A.: In adult patients with high-risk cervical spine injuries (P), should a screening computed tomography angiography (CTA) (I) versus no screening CTA (C) be performed to detect BCVI (O)?
- PICO question 2.B.: In adult patients with low-risk cervical spine injuries (P), should a screening CTA (I) versus no screening CTA (C) be performed to detect BCVI (O)?
- PICO question 3: In adult patients diagnosed with BCVI (P), should ATT (I) versus no ATT (C) be administered to decrease the incidence of stroke (O1) or mortality (O2)?
- PICO question 4: In adult patients with Grade II or III BCVIs (P), should routine endovascular stenting (as an adjunct to ATT) (I) versus ATT alone (C) be performed to reduce the risk of stroke (O1) or mortality (O2)?
Patients and Methods
Identification of References
Our systematic review was registered with the PROSPERO systematic registry of systematic reviews and meta-analyses (registration number CRD42018054777). An information specialist performed a systematic review of the literature using PubMed, Web of Science, EMBASE, and the Cochrane Library from inception (January 1965) to November 2017 using a combination of medical subject heading terms and keywords (Supplemental Digital Content 2, Supplementary Table 1, http://links.lww.com/TA/B601). The literature review was updated in November 2019. Exclusion criteria included non-English articles and case reports.
Selection of Outcomes
In accordance with the GRADE approach, outcomes were selected by the committee and voted on independently by each author in order of importance from 1 to 9, with scores of 7 to 9 representing critical outcomes.[35] Outcomes considered were as follows: stroke (hemorrhagic or ischemic), mortality, length of stay (hospital and intensive care unit), bleeding, infection, postprocedure complications, contrast-induced nephropathy, hospital costs, worsening of bleeding, need for delayed operative intervention, detection of injury, missed injury, and readmission. The critical outcome for PICO questions 1 and 2 was detection of BCVI. For PICO questions 3 and 4, the critical outcomes were stroke and mortality.
Data Extraction and Management
Following completion of the literature search, records were independently reviewed by two members of the guidelines group (D.Y.K., D.P.) to determine which records would proceed to title/abstract review followed by full-text review. Any conflicts regarding inclusion were resolved by a third member.
All references selected for full-text review were entered into a Microsoft Excel (version 16.17; Microsoft Corporation, Redmond, WA) spreadsheet to capture the following study variables: authors, article title, study design, interventions, and outcomes. All members of the PMG workgroup were provided with access to the full-text articles, grading resources, and instructions regarding risk of bias assessment.
Assessment of the Quality of Evidence
Evidence profile tables were generated for each PICO using GRADEpro Guideline Development Tool (McMaster University and Evidence Prime Inc., Hamilton, ON, Canada). The quality of evidence (QoE) for each critical outcome was determined on the basis of several explicit criteria including study limitations (risk of bias), publication bias, imprecision, inconsistency, and indirectness. Quality of evidence reflects the extent to which confidence in an estimate of the effect is adequate to support recommendations.[36] The GRADE approach results in an assessment of the quality of a body of evidence as high, moderate, low, or very low.
All members of the subcommittee voted on the proposed recommendations. The strength of a recommendation indicates the extent to which we can be confident that adherence to the recommendation will do more good than harm.[37] In addition to the overall QoE, members also considered the potential benefits and harms of a proposed management strategy or therapy, as well patients' values and preferences, and resource considerations.[38][39]
Statistical Analyses
Data from each study were entered into Review Manager (version 5.3; Cochrane Collaboration, London, United Kingdom) for quantitative analysis. A random effects model was used to calculate odds ratios (ORs) for categorical variables. Forest plots were generated to display effect estimates and confidence intervals (CIs) for individual studies. The proportion of variation between studies due to heterogeneity was quantified using the I[2] statistic and categorized as low (<40%), moderate (40–74%), or high (>75%).
Results
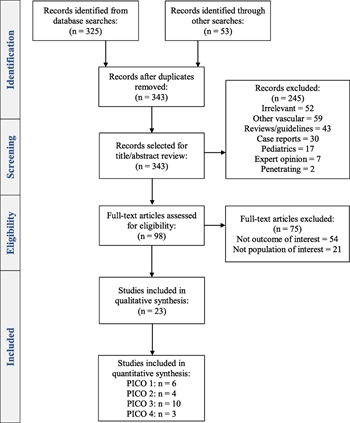
Figure 1: Preferred Reporting Items for Systematic Reviews and Meta-analyses diagram of included studies.
The search generated a total of 343 articles, of which 245 were excluded by title and abstract review. Of the remaining 98 articles, 75 were excluded following full-text review, thereby yielding 23 articles for analysis. The process for study selection is outlined in the Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram (Fig. 1).
Results for Screening Protocol to Detect BCVI (PICO 1)
In adult patients with blunt polytrauma, should a screening protocol versus no screening protocol be used to detect BCVI?
Qualitative Synthesis
Before the introduction of screening protocols, the incidence of BCVI was reported to be 0.1%,[1][15][40] and the diagnosis was often made following a stroke.[27][41] Currently, BCVI may be identified in up to 3% of polytrauma patients at centers that have developed, adopted, or implemented an institutional screening protocol.[5][42] The fundamental components of these protocols are similar, namely, to identify patients at risk for BCVI on the basis of established screening criteria (Table 1) and to perform either four-vessel biplanar cerebral arteriography (ART) or CTA to establish the diagnosis.
Implementation of BCVI screening protocols, development of expanded screening criteria,[5][43] and the use of whole-body computed tomography (CT) as a screening test[44] have all resulted in improved detection of asymptomatic BCVI, thereby allowing for earlier initiation of ATT and prevention of stroke and stroke-related mortality.[1][3][4][14][16][17][26][28][45] Furthermore, use of screening protocols has been demonstrated to be cost-effective.[18][46][47] Although it remains unknown how many centers have adopted screening criteria, a previous single-institutional study at a large, academic, tertiary care center demonstrated a lack of awareness of BCVI screening and grading criteria among physicians in an emergency setting.[48]
For the purposes of this PMG, studies that reported the incidence of BCVI detection preimplemetation and postimplementation of a screening protocol were identified. No direct comparisons were made between different screening criteria (Denver or Memphis) or screening modalities (ART or CTA). Although ART remains the criterion standard diagnostic test, several studies have demonstrated adequate sensitivity and specificity using CTA, particularly when a 16-channel or higher multidetector-row CT scanner is used.[49–52]
A total of six retrospective observational studies evaluated the effect of implementation of an institutional BCVI screening protocol on the detection of BCVI. In 1998, Biffl et al.[1] reported a BCVI incidence of 0.1% (12 of 12,429 patients) before aggressive screening using ART. Following institution of a screening protocol for asymptomatic patients, the incidence increased to 0.86%. In a 2001 study by Kerwin et al.,[2] the study authors sought to determine the incidence of abnormal ARTs ordered for liberalized screening of patients suspected to be at high-risk for BCVI. Before the protocol, the incidence of BCVI in 14,003 patients over a 12.5-year period was 0.03%. Postprotocol implementation, the incidence of BCVI was 1.1% for an 18-month period. Overall, 91% of all BCVIs were detected following the initiation of the aggressive screening protocol.
In 1999, Rogers et al.[15] reported their experience with cervical CTA as a screening modality for BCVI and found an overall incidence of 0.11%. There was a significant increase in the incidence of BCVI from 0.06% in the pre-CTA period to 0.19% in the post-CTA period (p = 0.02). A 2006 study[53] found a BCVI incidence of 1.4% in a CTA-based screening protocol cohort compared with a 0.17% detection rate in an unscreened population of blunt trauma patients. More recently,[54] incorporation of the Denver Screening Criteria into a diagnostic-imaging pathway using CTA demonstrated a significant increase in the incidence of BCVI from 0.52% (20 of 3,880 patients) to 1.06% (38 of 3,571 patients). A 2017 study[23] examining the effect of a consensus-based BCVI screening protocol with CTA on detection of BCVI found no difference in the preprotocol versus postprotocol incidence of BCVI (0.8% vs. 0.9%, p = 0.53).
Quantitative Synthesis (Meta-analysis)
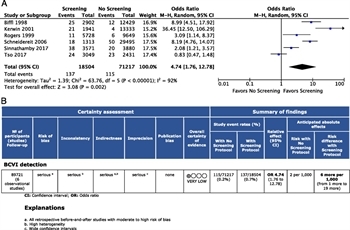
Figure 2: (A) Forest Plot for PICO 1 screening protocol versus no screening protocol for detection of BCVI. (B) GRADE profile for PICO 1 screening protocol versus no screening protocol for BCVI detection.
All six studies met the inclusion criteria for quantitative analysis.[1][2][15][23][53][54] Of the 89,721 patients studied, the proportion of patients diagnosed with BCVI using a screening protocol was 0.7% compared with 0.2% without the use of a screening protocol. The OR for detection of BCVI using a screening protocol was 4.7 (95% CI, 1.76–12.78; p = 0.002) (Fig. 2A). The I[2] was 92%, signifying substantial heterogeneity due to real differences in studies.[55]
Grading the Evidence
With the use of the GRADE framework for evaluating the data related to the outcome of detection of BCVI, the overall QoE was found to be very low because of the retrospective design of the studies with a serious risk of bias, inconsistency, indirectness, and imprecision (Fig. 2B). No evidence of publication bias was identified (Supplemental Digital Content 1, Supplementary Figure 1, http://links.lww.com/TA/B600).
Recommendation
In formulating a recommendation for PICO 1, we considered that most blunt polytrauma patients would benefit from and place a high value on a standardized approach to the detection of BCVI with a low potential for harm. Early diagnosis would allow for the initiation of ATT, which has been demonstrated to decrease the risk of stroke and death. We also considered that most patients would place significant value on avoiding such complications and accept the potential risks associated with CTA or ART. Based on this evidence, 15 authors (88%) voted for a strong recommendation, while 2 (12%) voted for a conditional recommendation. Thus, we recommend using a screening protocol for the detection of BCVI in adult patients with blunt polytrauma.
Results for Screening High- and Low-Risk Cervical Spine Injuries for BCVI (PICO 2)
2.A. In adult patients with high-risk cervical spine injuries (P), should a screening CTA (I) versus no screening CTA (C) be performed to detect BCVI (O)?
2.B. In adult patients with low-risk cervical spine injuries (P), should a screening CTA (I) versus no screening CTA (C) be performed to detect BCVI (O)?
Qualitative Synthesis
Cervical spine injuries remain the most common criterion leading to BCVI screening,[20] and the association between cervical spine injuries and BCVI, specifically vertebral artery injuries (VAIs), is well described.[16][56–59] Subluxation or distraction injuries,[60–63] vertebral foramen,[64] facet or transverse process fractures,[65][66] upper cervical spine fractures,[67][68] and the presence of a concomitant spinal cord injury,[62][69] have all been demonstrated to increase the risk for BCVI.
Early screening protocols emphasized the importance of screening all patients with cervical spine injuries as a significant proportion of patients may be found to harbor a BCVI.[14] As experience with the diagnosis and management of BCVI grew, a more selective approach to the diagnostic workup evolved, mainly as a result of the invasive nature, resource utilization, and potential complications associated with ART. Central to this selective approach was the identification of complex or high-risk cervical spine injuries. These injuries, as defined by the Denver group, include upper cervical spine (C1–C3) fractures, subluxation, and cervical spine fractures that extend into the transverse foramen.[70][71] This contrasts with low-risk cervical spine fracture patterns or injuries, which, as defined by the Memphis group, include any cervical spine injury.
With the widespread availability and improved accuracy of multidetector spiral CT, there has been a move once again toward a more liberal approach to performing CTA in patients with both high- and low-risk cervical spine injuries. This is based on the findings of previous studies demonstrating no predilection[17] or a similar incidence of BCVI in patients with and without high-risk criteria.[20][72–77]
Upon review of the literature, the PMG group did not identify any studies that specifically compared screening CTA with no screening CTA to detect BCVI among patients with either high- or low-risk cervical spine injuries. However, we did identify studies in which the incidence of VAIs was reported based on the presence of high- versus low-risk cervical spine injuries. Therefore, for the purposes of this PMG, studies were included for analysis if the incidence of BCVI was reported on the basis of the presence of high- versus low-risk cervical spine injuries.
In the largest study to date, Cothren et al.[71] performed screening ART in 766 patients, of whom 109 (14%) underwent screening for cervical spine fractures as the sole indication. Of 125 BCVI patients with cervical spine fractures, 117 (93.6%) were high risk. Among those with low-risk fractures, other screening criteria were fulfilled, which resulted in ART. A more recent study found that the presence of a high-risk cervical fracture was associated with a significantly increased risk for VAI on CTA (OR, 19.2; 95% CI, 2.6–139.9; p < 0.05).[78]
Among 328 patients in whom cervical spine fracture was the most common sole criteria for screening CTA, Emmett et al.[20] identified a 13% incidence of BCVI and no significant difference in the incidence of BCVI among patients with low-risk or “limited criteria” cervical fractures and high-risk fractures (15% vs. 9%, p = 0.16). The authors advocated for screening all cervical spine injuries, not just those deemed to be high risk. Similar recommendations were made by Kopelman et al.,[73] who proposed that all cervical spine fractures warrant screening for BCVI, despite only 2% of patients in the low-risk category being diagnosed with BCVI in their study.
Quantitative Synthesis (Meta-analysis)
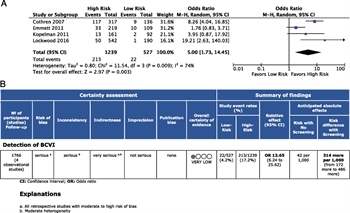
Figure 3: (A) Forest plot for PICO 2 low- versus high-risk cervical spine injuries and detection of BCVI. (B) GRADE profile for PICO 2 low- versus high-risk cervical spine injuries and BCVI detection
These same four studies were considered for quantitative analysis.[20][71][73][78] A total of 527 patients with low-risk cervical spine injuries were compared with 1,239 patients with high-risk injury patterns, yielding a total of 1,766 patients studied. Blunt cerebrovascular injury was detected in 22 patients (4.2%) with low-risk injuries and 213 (17.2%) with high-risk injuries. The OR for detection of BCVI among patients with high-risk cervical spine injuries was 12.7 (95% CI, 6.24–25.62; p = 0.003). There was moderate heterogeneity across studies (I[2] = 74%) (Fig. 3A).
Grading the Evidence
Within the GRADE framework for evaluating the data related to the detection of BCVI among patients with low- or high-risk cervical spine injuries, the overall QoE was found to be very low. There was a serious risk of bias insofar as all four studies were retrospective in design. Inconsistency and imprecision were also judged to be serious (Fig. 3B). Indirectness was deemed to be very serious, and publication bias was considered unlikely (Supplemental Digital Content 3, Supplementary Figure 2, http://links.lww.com/TA/B602).
Recommendation
In formulating a recommendation for PICO 2, we considered that the benefits of screening for BCVI in patients with high-risk cervical injuries outweigh the potential harms associated with imaging including, but not limited to, contrast-induced nephropathy. We also believe that most patients would place a high value on the early and rapid detection of BCVI and, if given the choice, most patients would prefer a noninvasive form of diagnosis such as CTA as opposed to ART. Based on this evidence, 12 authors (70.5%) voted for a strong recommendation, while 5 (29.5%) voted for a conditional recommendation. On the basis of these considerations, we recommend screening CTA in patients with high-risk cervical spine injuries to detect BCVI.
For patients with low-risk cervical spine injuries, the incidence of BCVI ranged from 2% to 9%. Given the potential morbidity of untreated BCVI and the widespread availability of CTA, we believe that the benefits of performing CTA in patients with low-risk cervical spine injuries may outweigh the potential risks. Furthermore, we also believe that the decision to perform imaging should be made on a case-by-case basis and that a thorough search for other criteria mandating screening for BCVI should be sought. Based on this evidence, 14 authors (82.3%) voted for a conditional recommendation, while 3 (17.7%) voted for a strong recommendation. On the basis of these considerations, we conditionally recommend BCVI screening in patients with low-risk cervical spine injuries to detect BCVI.
Results for ATT in Patients with BCVI (PICO 3)
In adult patients diagnosed with BCVI, should ATT versus no ATT be administered to prevent stroke (O1) or mortality (O2)?
Qualitative Synthesis
Before the development and application of BCVI screening criteria in the 1990s, the majority of patients with BCVI presented with symptoms and signs of neurologic ischemia.[1][16][26][27][79] Because the majority of BCVIs are inaccessible and operative repair is generally not feasible, treatment with systemic anticoagulation was introduced in an effort to decrease BCVI-related morbidity.[80][81] In 1996, Fabian et al.[26] were the first to demonstrate improved neurologic outcomes with the use of systemic anticoagulation in 62 patients with blunt carotid artery injuries (CAIs). Overall, 63% of survivors had a good neurologic outcome, and heparin therapy was also associated with improved survival. Recent literature demonstrates that institution of ATT (either systemic anticoagulation or antiplatelet medications) before the development of neurologic sequela has resulted in significantly improved outcomes with a reduction in BCVI-related stroke rates to less than 10%.[17][24][28][82]
For the purposes of this guideline, 10 comparative studies were identified, all of which were included in the construction of the evidence profile for stroke. We did not compare different types of ATT but rather grouped them into a single category as high-quality data regarding superiority of one agent over another are lacking.[8][12][79] Furthermore, in the majority of studies, more than one type of ATT was used thereby complicating any attempts to discern superiority of one agent over another. Finally, studies examining the impact of treatment type on outcomes have found no demonstrable difference in the risk of stroke or injury healing rates between groups.[12][17]
Five studies, including the 1996 study by Fabian et al.,[26] demonstrated a significant decrease in stroke incidence in patients treated with ATT. In four studies, ATT included systemic anticoagulation in the form of unfractionated heparin, antiplatelet agents, or both.[17][24][82][83] Subgroup analysis revealed no difference in the incidence of stroke between patients receiving systemic heparin and antiplatelet therapy.[17][24] In one of the only studies to examine the effect of treatment in patients with concomitant traumatic brain injury (TBI),[83] use of unfractionated heparin or aspirin (81 or 235 mg) resulted in a lower risk for stroke compared with no therapy (4% vs. 57%, p < 0.0001) with no increase in the risk of hemorrhagic deterioration based on pharmacologic exposure (5% vs. 6%, p = 0.6).
Five studies found no significant difference in stroke among patients who received or did not receive ATT.[1][16][30][84][85] Four of these studies examined only blunt CAIs, and one reported on outcomes in patients with VAIs. Stroke was variably defined across studies with outcomes stratified by severity of deficit[1][85] or outcome.[84] In a study of 38 patients with blunt VAIs, Biffl et al.[16] found a higher but nonsignificant incidence of deterioration in neurologic status from diagnosis to discharge in 60% of patients with VAIs not treated with systemic heparin versus 19% of those treated systemically (p = 0.11). In another study[30] of 65 patients being treated with ATT, 9% failed or suffered a cerebral infarction in the face of therapy, and the study authors found no difference in stroke rates between BCVI patients receiving antiplatelet therapy versus anticoagulation. In one study, bleeding complications were noted to be higher with systemic anticoagulation versus antiplatelet agents.[85]
For the critical outcome of mortality, six studies met the criteria.[1][16][26][30][83][84] The two largest studies[26][30] demonstrated a significant decrease in mortality in patients receiving ATT, whereas the remaining four studies found no difference.[1][16][83][84] Four studies reported on the incidence of BCVI-related mortality, which was found to occur in 8% to 76% of patients.[16][26][83][84]
Quantitative Synthesis (Meta-analysis)
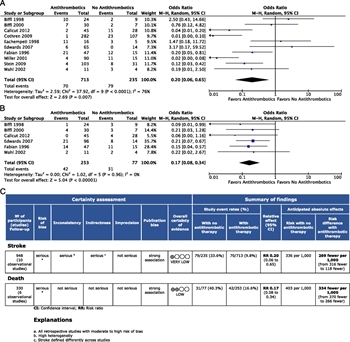
Figure 4: (A) Forest plot for PICO 3 ATT versus no ATT and stroke. (B) Forest plot for PICO 3 ATT versus no ATT and death. (C) GRADE profile for PICO 3 ATT versus no ATT and stroke or death.
All 10 studies met the criteria for quantitative analysis of stroke, and 6[17][24][26][83][84] met the criteria for mortality. A total of 713 patients received ATT and 235 did not. Strokes occurred in 9.8% of patients in the ATT group versus 33.6% of patients in the untreated group with an OR of 0.20 (95% CI, 0.06–0.65; p < 0.0001) (Fig. 4A). The I[2] was77%, falling into the “high” heterogeneity category.
For the outcome of mortality, 253 patients received ATT and 77 did not, for a total of 330 patients. Mortality was 16.6% in the ATT group compared with 40.4% in the non-ATT group, with an OR of 0.17 (95% CI, 0.08–0.34; p < 0.0001) (Fig. 4B). The I[2] was 0%, indicating minimal heterogeneity across studies examining mortality as an outcome.
Grading the Evidence
The overall QoE was found to be very low (stroke) to low (mortality) because of the retrospective design of the studies included with a serious risk of bias. For the outcome of stroke, both inconsistency and indirectness were found to be serious, whereas imprecision was not. For the outcome of mortality, inconsistency, indirectness, and imprecision were deemed not serious (Fig. 4C and D). No evidence of publication bias was identified (Supplemental Digital Content 4, Supplementary Figure 3, http://links.lww.com/TA/B603).
Recommendation
Despite the overall QoE being low (mortality) to very low (stroke), the panel considered that most patients with BCVI would likely benefit from the initiation of some form of ATT once the diagnosis has been confirmed. The benefits of ATT need to be weighed against the potential for bleeding complications and progression of hemorrhage, particularly among patients with TBI and solid organ injuries. The risks of initiating ATT seem to be low, and the use of ATT is associated with a significant reduction in both stroke and mortality.
Furthermore, we considered that many patients diagnosed with BCVI would place a high value on avoiding the potential morbidity and mortality associated with these injuries. Based on this evidence, 13 authors (76.5%) voted for a strong recommendation, while 4 (23.5%) voted for a conditional recommendation. On the basis of the available literature, we recommend the use of ATT to decrease the incidence of both stroke and mortality in patients with BCVI. This should be done as early and safely as possible following confirmation of the diagnosis and consideration should be given toward a multidisciplinary discussion of the optimal ATT among patients with concomitant injuries in whom therapy may exacerbate or worsen bleeding.
Results for Endovascular Stents in Grade II or III Injuries (PICO 4)
In adult patients with a Grade II or III BCVIs, should routine endovascular stenting (as an adjunct to ATT) versus ATT alone be performed to reduce the risk of stroke (O1) or mortality (O2)?
Qualitative Synthesis
The role of endovascular stenting for patients with BCVI is controversial. Early reports suggested a potential role for stenting[86] of Grade II injuries with significant luminal narrowing, as well as Grade III injuries (Table 2) to decrease the risk for embolism and rupture by decreasing flow into the pseudoaneurysm[87][88] for both CAIs[89–91] and VAIs.[92] Although early favorable results resulted in more widespread adoption of endovascular stenting, more recent studies demonstrate a potentially high risk for complications due to in-stent thrombosis, particularly in the absence of ATT,[1][93][94] and suggest that stent deployment only be used in cases where the injuries were persistent or enlarging. In a review of the literature DuBose et al.[95] identified, a 3.5% incidence of new neurologic deficits after stent placement. More recent data, however, have demonstrated improved outcomes in patients undergoing endovascular intervention for BCVIs compared with earlier series.[30][90]
For the purposes of this guideline, only studies that directly compared outcomes between patients with Grade II or III injuries who underwent endovascular stenting in the acute setting versus those treated with ATT alone were included in the analysis. Because of a paucity of data on mortality as an outcome, only the critical outcome of stroke was examined for this PICO.
In a 2011 study, DiCocco et al.[96] examined outcomes of endovascular treatment for 200 patients with angiographically confirmed BCVI, of which 80 asymptomatic patients underwent endovascular stenting in conjunction with antiplatelet therapy. Overall stroke rate was 16%. No difference in stroke rates was noted between patients who underwent medical management with ATT versus stenting even after stratified by grade and vessel injured.
Burlew et al.[97] compared ATT to endovascular stenting for patients with Grade II and III injuries. During the study period, the authors noted a decrease in the use of stents. The overall stroke rate in the stent versus no-stent groups was 8.7% versus 0.06% (p = 0.04) with no differences in the distribution of age, injury severity, or location of BCVI between groups. The study authors concluded that routine stenting is rarely necessary. A similar conclusion was reached by Shahan et al.[98] where the authors found a significant decrease in the use of stents over a 5-year period. Of 65 stents, the majority (61.5%) were placed for Grade III injuries. No significant difference in the risk for stroke was found between patients who underwent stenting with ATT versus ATT alone (3.1% vs. 2.4%) for Grade II or III injuries.
Quantitative Synthesis (Meta-analysis)
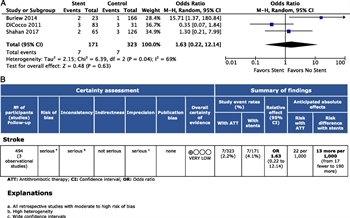
Figure 5: (A) Forest plot for PICO 4 endovascular therapy versus ATT and stroke. (B) GRADE profile for PICO 4 endovascular therapy versus ATT and stroke.
These same three studies were considered for quantitative analysis of endovascular stenting. A total of 171 patients underwent stenting of Grade II or III injuries, compared with 323 patients who did not, for a total of 494 patients studied. The stroke rate was 4.1% in patients who underwent endovascular stenting, with or without ATT, and 2.2% in those who received ATT alone. This difference was not statistically significant (OR, 1.63; 95% CI, 0.2–12.14; p = 0.63) (Fig. 5A). The I[2] statistic was 69%, signifying a moderate degree of heterogeneity.
Grading the Evidence
The overall QoE was found to be very low because of the retrospective design of the studies. In addition, risk of bias and imprecision were judged to be serious, whereas indirectness and inconsistency were deemed not serious (Fig. 5B). No evidence of publication bias was identified (Supplemental Digital Content 5, Supplementary Figure 4, http://links.lww.com/TA/B604).
Recommendation
In formulating a recommendation for PICO 4, we considered that despite the QoE being very low, the potential benefits of routine endovascular stenting of Grade II or III BCVIs do not outweigh the potential harms associated with this intervention including, but not limited, to stroke as a result of in-stent thrombosis. We also considered that most patients undergoing stent placement would require long-term antiplatelet therapy, and given that ATT alone has been demonstrated to decrease the risk for both stroke and mortality, the addition of an invasive procedure may not be warranted. Furthermore, the panel also considered that most patients would prefer to avoid an invasive procedure with its attendant complications and need for follow-up, repeat imaging, and unknown long-term outcomes. Based on this evidence, 15 members (88%) voted for a strong recommendation against, while 2 members (12%) voted for a conditional recommendation against. Thus, we recommend against the use of routine stenting as an adjunct to ATT in adult patients with Grade II or III BCVIs to reduce the risk of stroke.
Using These Guidelines in Clinical Practice
The recommendations formulated in this PMG represent the result of a comprehensive and systematic analysis of the scientific literature regarding the diagnosis and management of BCVI. These recommendations are meant to empower the decision-making process and should not replace clinical judgment. Unrecognized and untreated BCVI may result in significant clinical sequelae including stroke and death. Given the rarity of the injury and the lack of well-controlled prospective clinical trials, the level of evidence on which to base recommendations is low to very low. Despite the development of more liberal and expanded screening criteria, as many as 5% of patients who are found to have BCVI do not meet common screening criteria. More injuries will be found with more liberal screening, but there are diminishing returns for certain criteria (e.g., upper thoracic injuries) and lower energy mechanisms (e.g., ground-level falls). This applies to high- versus low-risk cervical injuries as well. Data support the use of ATT in patients diagnosed with BCVI, but there is no evidence to support a particular treatment. The use of various medications should be individualized. Currently, routine endovascular stent placement in the acute setting cannot be supported, but there may be individual cases (e.g., persistent or enlarging pseudoaneurysm) in which it would be considered appropriate and potentially beneficial to a patient.
Future Directions
Future studies should attempt to identify optimal screening criteria, with a more accurate estimate of the yield of various injury mechanisms and patterns. The search for the ideal screening test continues, and modalities such as whole-body CT scan should be further evaluated. In terms of ATT, future studies should compare therapies, and consider costs, duration, and need for follow-up imaging. The safety and optimal timing of initiation of ATT in patients with TBI and solid organ injuries remains to be defined. The role of endovascular stenting and other interventions (e.g., angioembolization) must be further clarified.
Conclusions
In summary, we propose four evidence-based recommendations regarding BCVI (Table 3). We recommend using a screening protocol to detect BCVI in adult polytrauma patients and performing screening CTA to detect BCVI in patients with high-risk cervical spine injuries. Among patients with low-risk cervical spine injuries, we conditionally recommend performing CTA to detect BCVI. In adult patients with BCVI, we recommend using ATT to prevent both stroke and mortality, and we recommend against the use of routine endovascular stenting in adult patients with Grade II or III BCVIs.
Authorship
All authors meet the authorship criteria for this article. All authors have seen and approved the final article as submitted. The first author (D.Y.K.) had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
D.Y.K., W.B., F.B., S.B., E.C., J.A.C., D.P., R.J., A.K., G.K., U.K., S.K., D.P., B.R.H.B., N.S., R.T., B.Y., and J.J.C. contributed in the conception and design. D.Y.K., W.B., F.B., S.B., E.C., J.A.C., D.P., R.J., A.K., G.K., U.K., S.K., D.P., B.R.H.B., N.S., R.T., B.Y., and J.J.C. contributed in the literature search. D.Y.K., W.B., F.B., S.B., E.C., J.A.C., D.P., R.J., A.K., G.K., U.K., S.K., D.P., B.R.H.B., N.S., R.T., B.Y., and J.J.C. contributed in the acquisition of data. D.Y.K., W.B., F.B., S.B., E.C., J.A.C., D.P., R.J., A.K., G.K., U.K., S.K., D.P., B.R.H.B., N.S., R.T., B.Y., and J.J.C. contributed in the analysis and interpretation of data. D.Y.K., W.B., F.B., S.B., E.C., J.A.C., D.P., R.J., A.K., G.K., U.K., S.K., D.P., B.R.H.B., N.S., R.T., B.Y., and J.J.C. contributed in the drafting of the article. D.Y.K., W.B., S.B., E.C., J.A.C., D.P., R.J., A.K., G.K., U.K., S.K., D.P., B.R.H.B., N.S., R.T., B.Y., and J.J.C. contributed in the critical revision of the article. D.Y.K., G.K., and J.J.C. contributed in the statistical expertise.
Acknowledgment
We thank Bethany Myers, information specialist at UCLA, for her expertise and assistance performing the literature search.
Disclosure
The authors declare no conflicts of interest.
References
- Biffl WL, Moore EE, Ryu RK, Offner PJ, Novak Z, Coldwell DM, Franciose RJ, Burch JM. The unrecognized epidemic of blunt carotid arterial injuries: early diagnosis improves neurologic outcome. Ann Surg. 1998;228(4):462–470. View Full Text| PubMed | CrossRef
- Kerwin AJ, Bynoe RP, Murray J, Hudson ER, Close TP, Gifford RR, Carson KW, Smith LP, Bell RM. Liberalized screening for blunt carotid and vertebral artery injuries is justified. J Trauma. 2001;51(2):308–314. View Full Text| PubMed | CrossRef
- Miller PR, Fabian TC, Croce MA, Cagiannos C, Williams JS, Vang M, Qaisi WG, Felker RE, Timmons SD. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg. 2002;236(3):386–393. View Full Text| PubMed | CrossRef
- Bruns BR, Tesoriero R, Kufera J, Sliker C, Laser A, Scalea TM, Stein DM. Blunt cerebrovascular injuryscreening guidelines: what are we willing to miss? J Trauma Acute Care Surg. 2014;76(3):691–695.
- Geddes AE, Burlew CC, Wagenaar AE, Biffl WL, Johnson JL, Pieracci FM, Campion EM, Moore EE. Expanded screening criteria for blunt cerebrovascular injury: a bigger impact than anticipated. Am J Surg. 2016;212(6):1167–1174. PubMed| CrossRef
- Crissey MM, Bernstein EF. Delayed presentation of carotid intimal tear following blunt craniocervical trauma. Surgery. 1974;75:543–549. PubMed
- Cogbill TH, Moore EE, Meissner M, et al. The spectrum of blunt injury to the carotid artery: a multicenter perspective. J Trauma. 1994;37(3):473–479. View Full Text| PubMed | CrossRef
- Biffl WL, Cothren CC, Moore EE, Kozar R, Cocanour C, Davis JW, McIntyre RC Jr., West MA, Moore FA. Western Trauma Association critical decisions in trauma: screening for and treatment of blunt cerebrovascular injuries. J Trauma. 2009;67(6):1150–1153. View Full Text| PubMed | CrossRef
- Fabian TC. Blunt cerebrovascular injuries: anatomic and pathologic heterogeneity create management enigmas. J Am Coll Surg. 2013;216(5):873–885. PubMed| CrossRef
- Fabian TC, George SM Jr., Croce MA, Mangiante EC, Voeller GR, Kudsk KA. Carotid artery trauma: management based on mechanism of injury. J Trauma. 1990;30(8):953–961. PubMed| CrossRef
- Biffl WL, Moore EE, Offner PJ, Brega KE, Franciose RJ, Burch JM. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47(5):845–853. View Full Text| PubMed | CrossRef
- Biffl WL, Ray CE Jr., Moore EE, Franciose RJ, Aly S, Heyrosa MG, Johnson JL, Burch JM. Treatment-related outcomes from blunt cerebrovascular injuries: importance of routine follow-up arteriography. Ann Surg. 2002;235(5):699–706. View Full Text| PubMed | CrossRef
- Burlew CC, Biffl WL. Blunt cerebrovascular trauma. Curr Opin Crit Care. 2010;16(6):587–595. View Full Text| PubMed | CrossRef
- Biffl WL, Moore EE, Offner PJ, Brega KE, Franciose RJ, Elliott JP, Burch JM. Optimizing screening for blunt cerebrovascular injuries. Am J Surg. 1999;178(6):517–522. PubMed| CrossRef
- Rogers FB, Baker EF, Osler TM, Shackford SR, Wald SL, Vieco P. Computed tomographic angiography as a screening modality for blunt cervical arterial injuries: preliminary results. J Trauma. 1999;46(3):380–385. View Full Text| PubMed | CrossRef
- Biffl WL, Moore EE, Elliott JP, Ray C, Offner PJ, Franciose RJ, Brega KE, Burch JM. The devastating potential of blunt vertebral arterial injuries. Ann Surg. 2000;231(5):672–681. View Full Text| PubMed | CrossRef
- Miller PR, Fabian TC, Bee TK, Timmons S, Chamsuddin A, Finkle R, Croce MA. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma. 2001;51(2):279–285. View Full Text| PubMed | CrossRef
- Cothren CC, Moore EE, Ray CE Jr., Ciesla DJ, Johnson JL, Moore JB, Burch JM. Screening for blunt cerebrovascular injuries is cost-effective. Am J Surg. 2005;190(6):845–849. PubMed
- Eastman AL, Muraliraj V, Sperry JL, Minei JP. CTA-based screening reduces time to diagnosis and stroke rate in blunt cervical vascular injury. J Trauma. 2009;67(3):551–556. View Full Text| PubMed | CrossRef
- Emmett KP, Fabian TC, DiCocco JM, Zarzaur BL, Croce MA. Improving the screening criteria for blunt cerebrovascular injury: the appropriate role for computed tomography angiography. J Trauma. 2011;70(5):1058–1063. View Full Text| PubMed | CrossRef
- Löhrer L, Vieth V, Nassenstein I, Hartensuer R, Niederstadt T, Raschke MJ, Vordemvenne T. Blunt cerebrovascular injuries in acute trauma care: a screening protocol. Eur Spine J. 2012;21(5):837–843. PubMed| CrossRef
- Jacobson LE, Ziemba-Davis M, Herrera AJ. The limitations of using risk factors to screen for blunt cerebrovascular injuries: the harder you look, the more you find. World J Emerg Surg. 2015;10:46.
- Tso MK, Lee MM, Ball CG, Morrish WF, Mitha AP, Kirkpatrick AW, Wong JH. Clinical utility of a screening protocol for blunt cerebrovascular injuryusing computed tomography angiography. J Neurosurg. 2017;126(4):1033–1041. PubMed | CrossRef
- Cothren CC, Biffl WL, Moore EE, Kashuk JL, Johnson JL. Treatment for blunt cerebrovascular injuries: equivalence of anticoagulation and antiplatelet agents. Arch Surg. 2009;144(7):685–690. View Full Text| PubMed | CrossRef
- Burlew CC, Sumislawski JJ, Behnfield CD, et al. Time to stroke: A Western Trauma Association multicenter study of blunt cerebrovascular injuries. J Trauma Acute Care Surg. 2018;85(5):858–866.
- Fabian TC, Patton JH Jr., Croce MA, Minard G, Kudsk KA, Pritchard FE. Blunt carotid injury. Importance of early diagnosis and anticoagulant therapy. Ann Surg. 1996;223(5):513–522. View Full Text| PubMed | CrossRef
- Ahmad HA, Gerraty RP, Davis SM, Cameron PA. Cervicocerebral artery dissections. J Accid Emerg Med. 1999;16(6):422–424. PubMed| CrossRef
- Kraus RR, Bergstein JM, DeBord JR. Diagnosis, treatment, and outcome of blunt carotid arterial injuries. Am J Surg. 1999;178(3):190–193. PubMed| CrossRef
- Cothren CC, Moore EE, Biffl WL, Ciesla DJ, Ray CE Jr., Johnson JL, Moore JB, Burch JM. Anticoagulation is the gold standard therapy for blunt carotid injuries to reduce stroke rate. Arch Surg. 2004;139(5):540–545. View Full Text| PubMed | CrossRef
- Edwards NM, Fabian TC, Claridge JA, Timmons SD, Fischer PE, Croce MA. Antithrombotic therapy and endovascular stents are effective treatment for blunt carotid injuries: results from longterm followup. J Am Coll Surg. 2007;204(5):1007–1013. PubMed| CrossRef
- Shahan CP, Magnotti LJ, McBeth PB, Weinberg JA, Croce MA, Fabian TC. Early antithrombotic therapy is safe and effective in patients with blunt cerebrovascular injuryand solid organ injury or traumatic brain injury. J Trauma Acute Care Surg. 2016;81(1):173–177.
- Berne JD, Norwood SH, McAuley CE, Vallina VL, Creath RG, McLarty J. The high morbidity of blunt cerebrovascular injuryin an unscreened population: more evidence of the need for mandatory screening protocols. J Am Coll Surg. 2001;192(3):314–321. PubMed | CrossRef
- Bromberg WJ, Collier BC, Diebel LN, Dwyer KM, Holevar MR, Jacobs DG, Kurek SJ, Schreiber MA, Shapiro ML, Vogel TR. Blunt cerebrovascular injurypractice management guidelines: the Eastern Association for the Surgery of Trauma. J Trauma. 2010;68(2):471–477. View Full Text | PubMed
- Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, Dahm P, Eastern Association for the Surgery of Trauma Practice Management Guidelines Ad Hoc Committee. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S283–S287.
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction- GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. PubMed| CrossRef
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, Alderson P, Glasziou P, Falck-Ytter Y, Schünemann HJ. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64(4):395–400. PubMed| CrossRef
- Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. View Full Text| PubMed | CrossRef
- Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66(7):719–725. PubMed| CrossRef
- Andrews JC, Schünemann HJ, Oxman AD, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation's direction and strength. J Clin Epidemiol. 2013;66(7):726–735. PubMed| CrossRef
- Davis JW, Holbrook TL, Hoyt DB, Mackersie RC, Field TO Jr., Shackford SR. Blunt carotid artery dissection: incidence, associated injuries, screening, and treatment. J Trauma. 1990;30(12):1514–1517. View Full Text| PubMed | CrossRef
- Carrillo EH, Osborne DL, Spain DA, Miller FB, Senler SO, Richardson JD. Blunt carotid artery injuries: difficulties with the diagnosis prior to neurologic event. J Trauma. 1999;46(6):1120–1125. View Full Text| PubMed | CrossRef
- Bonatti M, Vezzali N, Ferro F, Manfredi R, Oberhofer N, Bonatti G. Blunt cerebrovascular injury: diagnosis at whole-body MDCT for multi-trauma. Insights Imaging. 2013;4(3):347–355.
- Burlew CC, Biffl WL, Moore EE, Barnett CC, Johnson JL, Bensard DD. Blunt cerebrovascular injuries: redefining screening criteria in the era of noninvasive diagnosis. J Trauma Acute Care Surg. 2012;72(2):330–335.
- Laser A, Kufera JA, Bruns BR, Sliker CW, Tesoriero RB, Scalea TM, Stein DM. Initial screening test for blunt cerebrovascular injury: validity assessment of whole-body computed tomography. Surgery. 2015;158(3):627–635. View Full Text| PubMed | CrossRef
- Biffl WL, Moore EE, Offner PJ, Burch JM. Blunt carotid and vertebral arterial injuries. World J Surg. 2001;25:1036–1043. PubMed| CrossRef
- Kaye D, Brasel KJ, Neideen T, Weigelt JA. Screening for blunt cerebrovascular injuries is cost-effective. J Trauma. 2011;70(5):1051–1056. View Full Text| PubMed | CrossRef
- Wu X, Malhotra A, Forman HP, Nunez D, Sanelli P. The use of high-risk criteria in screening patients for blunt cerebrovascular injury: a survey. Acad Radiol. 2017;24(4):456–461. PubMed| CrossRef
- Malhotra A, Wu X, Kalra VB, Schindler J, Matouk CC, Forman HP. Evaluation for blunt cerebrovascular injury: review of the literature and a cost-effectiveness analysis. AJNR Am J Neuroradiol. 2016;37(2):330–335. PubMed| CrossRef
- Berne JD, Reuland KS, Villarreal DH, McGovern TM, Rowe SA, Norwood SH. Sixteen-slice multi-detector computed tomographic angiography improves the accuracy of screening for blunt cerebrovascular injury. J Trauma. 2006;60:1204–1209. View Full Text| PubMed | CrossRef
- Eastman AL, Chason DP, Perez CL, McAnulty AL, Minei JP. Computed tomographic angiography for the diagnosis of blunt cervical vascular injury: is it ready for primetime? J Trauma. 2006;60:925–929. View Full Text| PubMed | CrossRef
- Biffl WL, Egglin T, Benedetto B, Gibbs F, Cioffi WG. Sixteen-slice computed tomographic angiography is a reliable noninvasive screening test for clinically significant blunt cerebrovascular injuries. J Trauma. 2006;60:745–751. View Full Text| PubMed | CrossRef
- Paulus EM, Fabian TC, Savage SA, Zarzaur BL, Botta V, Dutton W, Croce MA. Blunt cerebrovascular injuryscreening with 64-channel multidetector computed tomography: more slices finally cut it. J Trauma Acute Care Surg. 2014;76(2):279–283.
- Schneidereit NP, Simons R, Nicolaou S, Graeb D, Brown DR, Kirkpatrick A, Redekop G, McKevitt EC, Neyestani A. Utility of screening for blunt vascular neck injuries with computed tomographic angiography. J Trauma. 2006;60(1):209–215. View Full Text| PubMed | CrossRef
- Sinnathamby M, Rao SV, Weber DG. Increased detection of blunt carotid and vertebral artery injuryafter implementation of diagnostic imaging pathway in level 1 trauma centre in Western Australia. Injury. 2017;48(9):1917–1921. PubMed | CrossRef
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. View Full Text| PubMed | CrossRef
- Franz RW, Willette PA, Wood MJ, Wright ML, Hartman JF. A systematic review and meta-analysis of diagnostic screening criteria for blunt cerebrovascular injuries. J Am Coll Surg. 2012;214(3):313–327. PubMed| CrossRef
- Lebl DR, Bono CM, Velmahos G, Metkar U, Nguyen J, Harris MB. Vertebral artery injuryassociated with blunt cervical spine trauma: a multivariate regression analysis. Spine (Phila Pa 1976). 2013;38(16):1352–1361. View Full Text | PubMed | CrossRef
- Cook A, Osler T, Gaudet M, Berne J, Norwood S. Blunt cerebrovascular injuryis poorly predicted by modeling with other injuries: analysis of NTDB data. J Trauma. 2011;71(1):114–119. View Full Text | PubMed | CrossRef
- Berne JD, Cook A, Rowe SA, Norwood SH. A multivariate logistic regression analysis of risk factors for blunt cerebrovascular injury. J Vasc Surg. 2010;51(1):57–64. PubMed| CrossRef
- Vilela MD, Kim LJ, Bellabarba C, Bransford RJ. Blunt cerebrovascular injuries in association with craniocervical distraction injuries: a retrospective review of consecutive cases. Spine J. 2015;15(3):499–505. PubMed| CrossRef
- Gupta P, Kumar A, Gamangatti S. Mechanism and patterns of cervical spine fractures-dislocations in vertebral artery injury. J Craniovertebr Junction Spine. 2012;3(1):11–15.
- Nakajima H, Nemoto M, Torio T, Takeda R, Ooigawa H, Araki R, Kurita H. Factors associated with blunt cerebrovascular injuryin patients with cervical spine injury. Neurol Med Chir (Tokyo). 2014;54(5):379–386. PubMed | CrossRef
- Willis BK, Greiner F, Orrison WW, Benzel EC. The incidence of vertebral artery injuryafter midcervical spine fracture or subluxation. Neurosurgery. 1994;34(3):435–441. View Full Text | PubMed | CrossRef
- McKinney A, Ott F, Short J, McKinney Z, Truwit C. Angiographic frequency of blunt cerebrovascular injuryin patients with carotid canal or vertebral foramen fractures on multidetector CT. Eur J Radiol. 2007;62(3):385–393. PubMed | CrossRef
- Chung D, Sung JK, Cho DC, Kang DH. Vertebral artery injuryin destabilized midcervical spine trauma; predisposing factors and proposed mechanism. Acta Neurochir. 2012;154(11):2091–2098. PubMed | CrossRef
- Bonney PA, Burks JD, Conner AK, Glenn CA, Baker CM, Cheema AA, Archer JB, Buster BE, Albrecht RM, Bohnstedt BN. Vertebral artery injuryin patients with isolated transverse process fractures. J Clin Neurosci. 2017;41:111–114. PubMed | CrossRef
- Payabvash S, McKinney AM, McKinney ZJ, Palmer CS, Truwit CL. Screening and detection of blunt vertebral artery injuryin patients with upper cervical fractures: the role of cervical CT and CT angiography. Eur J Radiol. 2014;83(3):571–577. PubMed | CrossRef
- Hwang PY, Lewis PM, Balasubramani YV, Madan A, Rosenfeld JV. The epidemiology of BCVI at a single state trauma Centre. Injury. 2010;41(9):929–934. PubMed| CrossRef
- Torina PJ, Flanders AE, Carrino JA, Burns AS, Friedman DP, Harrop JS, Vacarro AR. Incidence of vertebral artery thrombosis in cervical spine trauma: correlation with severity of spinal cord injury. AJNR Am J Neuroradiol. 2005;26(10):2645–2651. PubMed
- Cothren CC, Moore EE, Biffl WL, Ciesla DJ, Ray CE Jr., Johnson JL, Moore JB, Burch JM. Cervical spine fracture patterns predictive of blunt vertebral artery injury. J Trauma. 2003;55(5):811–813. View Full Text| PubMed | CrossRef
- Cothren CC, Moore EE, Ray CE Jr., Johnson JL, Moore JB, Burch JM. Cervical spine fracture patterns mandating screening to rule out blunt cerebrovascular injury. Surgery. 2007;141(1):76–82. View Full Text| PubMed | CrossRef
- Ringer AJ, Matern E, Parikh S, Levine NB. Screening for blunt cerebrovascular injury: selection criteria for use of angiography. J Neurosurg. 2010;112(5):1146–1149. PubMed| CrossRef
- Kopelman TR, Leeds S, Berardoni NE, O'Neill PJ, Hedayati P, Vail SJ, Pieri PG, Feiz-Erfan I, Singer Pressman MA. Incidence of blunt cerebrovascular injuryin low-risk cervical spine fractures. Am J Surg. 2011;202(6):684–688. PubMed | CrossRef
- Grabowski G, Robertson RN, Barton BM, Cairns MA, Webb SW. Blunt cerebrovascular injuryin cervical spine fractures: are more-Liberal screening criteria warranted? Global Spine J. 2016;6(7):679–685.
- Fleck SK, Langner S, Baldauf J, Kirsch M, Rosenstengel C, Schroeder HW. Blunt craniocervical artery injury in cervical spine lesions: the value of CT angiography. Acta Neurochir. 2010;152(10):1679–1686. PubMed| CrossRef
- Drain JP, Weinberg DS, Ramey JS, Moore TA, Vallier HA. Indications for CT-angiography of the vertebral arteries after trauma. Spine (Phila Pa 1976). 2018;43(9):E520–E524. View Full Text| PubMed | CrossRef
- Berne JD, Norwood SH. Blunt vertebral artery injuries in the era of computed tomographic angiographic screening: incidence and outcomes from 8,292 patients. J Trauma. 2009;67(6):1333–1338. View Full Text| PubMed | CrossRef
- Lockwood MM, Smith GA, Tanenbaum J, Lubelski D, Seicean A, Pace J, Benzel EC, Mroz TE, Steinmetz MP. Screening via CT angiogram after traumatic cervical spine fractures: narrowing imaging to improve cost effectiveness. Experience of a level I trauma center. J Neurosurg Spine. 2016;24(3):490–495. PubMed| CrossRef
- Cothren CC, Moore EE. Blunt Cerebrovascular Injuries. Clinics. 2005;60(6):489–496.
- Parikh AA, Luchette FA, Valente JF, Johnson RC, Anderson GL, Blebea J, Rosenthal GJ, Hurst JM, Johannigman JA, Davis K Jr. Blunt carotid artery injuries. J Am Coll Surg. 1997;185(1):80–86. PubMed| CrossRef
- Colella JJ, Diamond DL. Blunt carotid injury: reassessing the role of anticoagulation. Am Surg. 1996;62(3):212–217 PubMed
- Stein DM, Boswell S, Sliker CW, Lui FY, Scalea TM. Blunt cerebrovascular injuries: does treatment always matter? J Trauma. 2009;66(1):132–143. View Full Text| PubMed | CrossRef
- Callcut RA, Hanseman DJ, Solan PD, Kadon KS, Ingalls NK, Fortuna GR, Tsuei BJ, Robinson BR. Early treatment of blunt cerebrovascular injurywith concomitant hemorrhagic neurologic injury is safe and effective. J Trauma Acute Care Surg. 2012;72(2):338–345.
- Wahl WL, Brandt MM, Thompson BG, Taheri PA, Greenfield LJ. Antiplatelet therapy: an alternative to heparin for blunt carotid injury. J Trauma. 2002;52(5):896–901. View Full Text| PubMed | CrossRef
- Eachempati SR, Vaslef SN, Sebastian MW, Reed RL 2nd. Blunt vascular injuries of the head and neck: is heparinization necessary? J Trauma. 1998;45(6):997–1004. View Full Text| PubMed | CrossRef
- Duke BJ, Ryu RK, Coldwell DM, Brega KE. Treatment of blunt injury to the carotid artery by using endovascular stents: an early experience. J Neurosurg. 1997;87(6):825–829. PubMed| CrossRef
- Coldwell DM, Novak Z, Ryu RK, Brega KE, Biffl WL, Offner PJ, Franciose RJ, Burch JM, Moore EE. Treatment of posttraumatic internal carotid arterial pseudoaneurysms with endovascular stents. J Trauma. 2000;48(3):470–472. View Full Text| PubMed | CrossRef
- Lu CJ, Kao HL, Sun Y, Liu HM, Jeng JS, Yip PK, Lee YT. The hemodynamic effects of internal carotid artery stenting: a study with color-coded duplex sonography. Cerebrovasc Dis. 2003;15(4):264–269. View Full Text| PubMed | CrossRef
- Tsai YH, Wong HF, Weng HH, Chen YL. Stent-graft treatment of traumatic carotid artery dissecting pseudoaneurysm. Neuroradiology. 2010;52(11):1011–1016. PubMed| CrossRef
- Seth R, Obuchowski AM, Zoarski GH. Endovascular repair of traumatic cervical internal carotid artery injuries: a safe and effective treatment option. AJNR Am J Neuroradiol. 2013;34(6):1219–1226. PubMed| CrossRef
- Berne JD, Reuland KR, Villarreal DH, McGovern TM, Rowe SA, Norwood SH. Internal carotid artery stenting for blunt carotid artery injuries with an associated pseudoaneurysm. J Trauma. 2008;64(2):398–405. View Full Text| PubMed | CrossRef
- Lee YJ, Ahn JY, Han IB, Chung YS, Hong CK, Joo JY. Therapeutic endovascular treatments for traumatic vertebral artery injuries. J Trauma. 2007;62(4):886–891. View Full Text| PubMed | CrossRef
- Biffl WL, Moore EE, Ray C, Elliott JP. Emergent stenting of acute blunt carotid artery injuries: a cautionary note. J Trauma. 2001;50(5):969–971. View Full Text| PubMed | CrossRef
- Cothren CC, Moore EE, Ray CE Jr., Ciesla DJ, Johnson JL, Moore JB, Burch JM. Carotid artery stents for blunt cerebrovascular injury: risks exceed benefits. Arch Surg. 2005;140(5):480–485. View Full Text| PubMed | CrossRef
- DuBose J, Recinos G, Teixeira PG, Inaba K, Demetriades D. Endovascular stenting for the treatment of traumatic internal carotid injuries: expanding experience. J Trauma. 2008;65(6):1561–1566. View Full Text| PubMed | CrossRef
- DiCocco JM, Fabian TC, Emmett KP, et al. Optimal outcomes for patients with blunt cerebrovascular injury(BCVI): tailoring treatment to the lesion. J Am Coll Surg. 2011;212(4):549–557. PubMed | CrossRef
- Burlew CC, Biffl WL, Moore EE, Pieracci FM, Beauchamp KM, Stovall R, Wagenaar AE, Jurkovich GJ. Endovascular stenting is rarely necessary for the management of blunt cerebrovascular injuries. J Am Coll Surg. 2014;218(5):1012–1017. PubMed| CrossRef
- Shahan CP, Sharpe JP, Stickley SM, Manley NR, Filiberto DM, Fabian TC, Croce MA, Magnotti LJ. The changing role of endovascular stenting for blunt cerebrovascular injuries. J Trauma Acute Care Surg. 2018;84(2):308–311.
Keywords: Blunt cerebrovascular injury; carotid artery injury; vertebral artery injury; antithrombotic; stent


