Blunt Force Bladder Injuries Management of
Published 2019
Citation: J Trauma. 86(2):326-336, Feb 2019
Authors
Yeung, Lawrence L., MD; McDonald, Amy A., MD; Como, John J., MD, MPH; Robinson, Bryce, MD; Knight, Jennifer, MD; Person, Michael A., MD; Lee, Jane K., MD; Dahm, Philipp, MD, MHSc
Author Information
From the Department of Urology (L.L.Y.), College of Medicine, University of Florida, Gainesville, Florida; Department of Surgery (A.A.M), Cleveland VAMC, Case Western Reserve University; Department of Surgery (J.J.C.), MetroHealth Medical Center, Cleveland, Ohio; Department of Surgery (B.R.), Harborview Medical Center, University of Washington, Seattle, Washington; Department of Surgery (J.K.), West Virginia University, Morgantown, West Virginia; Department of Surgery (M.A.P.), University of South Dakota, Sanford School of Medicine, Vermillion, South Dakota; Department of Surgery (J.K.L.), University of Illinois at Chicago, Illinois; and Department of Urology (P.D.), Minneapolis VAMC, University of Minnesota, Minneapolis, Minnesota.
Submitted: August 29, 2018, Revised: August 18, 2018, Accepted: October 30, 2018, Published online: November 28, 2018.
This work was presented in part at the 27th annual meeting of the Eastern Association for the Surgery of Trauma, January 14–18, 2014, in Naples, Florida, and at the 31st annual meeting of the Eastern Association for the Surgery of Trauma, January 9–13, 2018, in Orlando, Florida.
Address for reprints: Lawrence L. Yeung, MD, 1600 SW Archer Rd, Box 100247, Gainesville, FL 32610; email: lawrence.yeung@urology.ufl.edu.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
Abstract
BACKGROUND The diagnostic evaluation and clinical management of bladder injuries caused by blunt force trauma are variable. We aim to formulate a practice management guideline using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.
METHODS The working group, patient, intervention, comparator, outcome (PICO), formulated four questions regarding the following topics: (1) diagnostic evaluation based on patient baseline risk of bladder injury (computed tomography cystography vs. no imaging); (2) management of intraperitoneal bladder injuries (operative versus nonoperative); (3) management of extraperitoneal bladder injuries based on complexity of injury (operative vs. nonoperative); and (4) diagnostic follow-up of bladder injuries based on complexity of repair (cystography vs. no cystography). A systematic review of the MEDLINE database for English language articles with adult patients was undertaken. RevMan 5 (Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and GRADEpro (GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015) software were used. Recommendations were voted on by working group members. Consensus was obtained for each recommendation.
RESULTS Three hundred ninety-three articles were screened, resulting in a full-text review of 64 articles. Seventeen articles were used to formulate the recommendations of this guideline. Several recommendations are made. The need for initial computed tomography cystography after trauma depends on characteristics of the trauma itself, but it is not recommended in patients without gross hematuria. In general, patients with intraperitoneal bladder ruptures should undergo operative repair. This is not routinely necessary in those with extraperitoneal ruptures unless the injury is complex. The need for follow-up cystography after bladder repair depends on the risk of urine leak. Those with low risk of urine leak do not require a follow-up study.
CONCLUSION Using the GRADE process, the panel made nine recommendations based on four PICO questions concerning the evaluation and management of blunt force bladder injuries.
Blunt external trauma, from either a direct blow to the abdomen or shearing forces from a pelvic fracture, accounts for the majority of bladder injuries presenting in emergency rooms. Bladder injuries are associated with multiple organ injuries making mortality rates associated with bladder injuries as high as 22%. Overall, roughly 60% of injuries are extraperitoneal, 30% intraperitoneal, and 10% occur concomitantly.[1]
The purpose of this practice management guideline was to evaluate critical clinical questions regarding the diagnosis and management of bladder trauma resulting from blunt abdominoperineal trauma. Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology was used to provide evidence-based guidance.[2] Throughout this guideline, we define bladder injuries/ruptures as being full thickness bladder lacerations/injuries. Typically, these injuries are repaired in two layers with absorbable sutures because of their nonlithogenic property.
Objectives
To develop this guideline, we assembled a panel composed of experts in the field of urology, traumatology, and GRADE methodology. We defined four population (P), intervention (I), comparator (C), and outcome (O) (PICO) questions a priori to the systematic review. These PICO questions were derived through a process panel deliberation:
- PICO 1: In patients with blunt abdominal/pelvic trauma (P), should retrograde computed tomography (CT) cystography (I) versus no imaging study be used to diagnose bladder injuries (O)?
- PICO 2: In patients sustaining blunt abdominopelvic trauma with intraperitoneal bladder rupture (P), should operative repair (I) versus nonoperative management (C) be used to decrease complications from the bladder injury (O)?
- PICO 3: In patients sustaining blunt abdominopelvic trauma with extraperitoneal bladder rupture (P), should operative repair (I) versus nonoperative management (C) be used to decrease complications from the bladder injury (O)?
- PICO 4: In patients who have undergone operative or nonoperative management of bladder rupture (P), should cystography (I) versus no imaging study (C) be used to evaluate for bladder closure (O)?
Identification of References
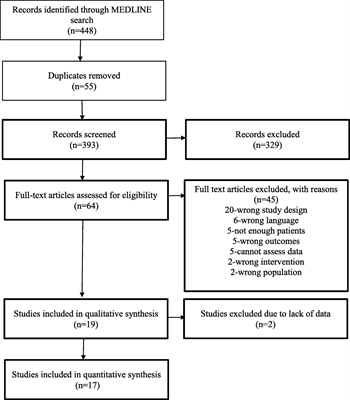
With the assistance of a professional medical librarian, a systematic review of the medical literature was performed. The MEDLINE database was searched to identify English-language human studies published from January 1975 to July 2016 using the medical subject headings and keywords listed (Table, Supplemental Digital Content 1). All randomized controlled trials, observational studies, and retrospective studies were considered. Studies of adult patients (age, ≥18 years) sustaining blunt abdominal/pelvic trauma were included. Letters to the editor, book chapters, reviewed articles, studies on pediatric patients, penetrating trauma, and case series of less than 20 patients were excluded. Three authors performed the title and abstract review of the literature for each PICO question. One author (L.L.Y.) then performed the full text review of the remaining articles (Fig. 1).
Outcome Measure Types
The relevant outcome measures for each PICO question were established a priori. This was a two-stage process. Panel members identified the most relevant outcomes relating to each PICO question. A nine-point scale, as described by the GRADE methodology, was then used by the panel to rate the outcomes in terms of importance.[3] Outcomes with scores of 7 to 9 were considered critical to the decision making process and were included in the review. For PICO 1, the critical outcomes were the true positives (patients with bladder rupture), true negatives (patients without bladder rupture), false negatives (patients incorrectly classified as not having bladder rupture), and false positives (patients incorrectly classified as having bladder rupture). For PICO 2, the critical outcomes were overall survival, successful bladder closure, and infectious complications. For PICO 3, the critical outcomes were overall survival, infectious complications, and need for operative repair. Infectious complications in PICO 2 and 3 included sepsis secondary to urinary tract infection, infected pelvic hardware, osteomyelitis, and perivesical space abscess formation. For PICO 4, the critical outcomes were the true positives (patients with urine leak), true negatives (patients without urine leakage), false negatives (patients incorrectly classified as not having urine leakage), and false positives (patients incorrectly classified as having urine leakage).
Data Extraction and Methodology
Data were extracted from each study performed using a standardized data collection sheet, which we pilot tested. Data from each study were entered into Review Manager (Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) for the meta-analysis. The primary author checked all entered data in duplicate to ensure accuracy. Forest plots were generated for each critical outcome, and risk ratios were calculated as measures of effect for dichotomous outcomes. The I [2] statistic was used to determine the degree of heterogeneity between studies. All studies were analyzed using a random effects model.
We used the Newcastle-Ottawa Scale to assess the risk of bias for observational studies.[4] We used GRADE to assess the quality of evidence on a per outcome basis considering study limitations (risk of bias), inconsistency, indirectness, imprecision, and publication bias. The Quality Assessment of Diagnostic Accuracy Studies 2 tool was used to assess the quality of the diagnostic accuracy studies.[5]
Evidence profiles were created for each PICO using the GRADEpro GDT software (GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015). All members of the committee voted on the proposed recommendations via teleconference.
The evidence to decision framework was used by the panel for each PICO. This took into consideration the quality of evidence, relationship of benefits and harms, patient values and preferences (as represented by panel participants), and resource utilization when arriving at a recommendation.
The PICOs 1 and 4 are diagnostic questions so the recommendations are presented for low, moderate, and high-risk groups.
Results for CT Cystography (PICO 1)
Qualitative Synthesis
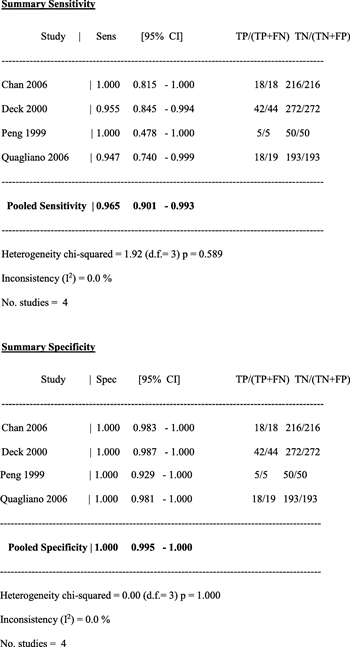
Radiographic detection of bladder injury can be performed by retrograde filling of the bladder with contrast followed by plain film imaging and postdrainage films (plain film cystography) or retrograde filling of the bladder with contrast followed by CT imaging (CT cystography). Four studies (two prospective cohort and two retrospective cohort) assessed the utility of retrograde CT cystography in diagnosing bladder rupture in patients sustaining blunt abdominopelvic trauma, and the data from these studies pooled to determine the overall sensitivity and specificity in diagnosing bladder injury.[6–9] In the prospective study by Quagliano et al.,[7]CT cystography was found to have equivalent sensitivity (95%) and specificity (100%) as plain film cystographyat detecting the presence or absence of bladder injury. Chan et al.[9] performed a retrospective review and compared CT cystography to intraoperative findings, clinical follow-up, or both, and the sensitivity and specificity of CT cystography in diagnosing bladder rupture were each 100%. Deck et al.[6] performed a retrospective review of 316 patients who underwent CT cystography and compared the radiographic findings to operative findings. The overall sensitivity and specificity of CT cystography in diagnosing bladder rupture were found to be 95% and 100%, respectively. In the prospective series by Peng et al.,[8] 55 patients with hematuriaand blunt abdominal trauma were screened with CT cystography, and 5 were identified with bladder injuries that were confirmed intraoperatively. The 50 patients with negative CT cystograms underwent conventional cystography, and no other bladder injuries were diagnosed, giving CT cystography a sensitivity of 100% and specificity of 100% at diagnosing bladder injury.
Two studies (one retrospective cohort and one prospective cohort) were used to determine the incidence of bladder rupture in three groups of patients: low risk (pelvic fracture and microhematuria), medium risk (gross hematuria), and high risk (gross hematuria and pelvic fracture) groups.[10][11] The study performed by Morey et al.,[10] was a systematic review of a contemporary retrospective series of patients with blunt bladder trauma. The prevalence of bladder rupture in patients with pelvic fracture and microhematuria (low likelihood group) was 0.6%, while the prevalence of bladder rupture in patients with pelvic fracture and gross hematuria (high likelihood group) was 29%. In the study by Brewer et al.,[11] patients sustaining blunt abdominopelvic trauma with hematuria were prospectively evaluated, and the incidence of bladder rupture in patients with gross hematuria (moderate likelihood group) was 21%. The pooled sensitivity and specificity analysis for CT cystography was then applied to determine the utility of retrograde CT cystography for each of these likelihood groups.
Quantitative Synthesis
In the four studies (including a total of 817 patients) used to calculate the sensitivity and specificity of CT cystography, the pooled overall sensitivity was 0.965 (95% confidence interval [CI], 0.90–0.99) and specificity was 1.00 (95% CI, 0.99–1.00)[6–9] (Fig. 2).
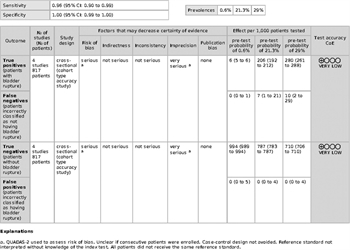
When the pooled sensitivity and specificity of CT cystography are applied to the low likelihood (0.6%) of bladder injury group, 6 of 1,000 people would be correctly diagnosed as having a bladder rupture (true positive). None of the patients would be incorrectly diagnosed as not having a bladder rupture (false negative), 994 of 1,000 would be correctly diagnosed as not having a bladder rupture (true negative), and none of the patients would be incorrectly classified as having a bladder rupture (false positive) (Figure, Supplemental Digital Content 1, http://links.lww.com/TA/B240).
For the moderate likelihood group (21.3%), 206 of 1,000 people would be diagnosed correctly as having a bladder rupture (true positive). Seven patients would be diagnosed incorrectly as not having a bladder rupture (false negative), and 787 of 1,000 would be diagnosed correctly as not having a bladder rupture (true negative). None of the patients would be classified incorrectly as having a bladder rupture (false positive) (Figure, Supplemental Digital Content 2, http://links.lww.com/TA/B241).
For the high likelihood group (29%), 280 of 1,000 people would be diagnosed correctly as having a bladder rupture (true positive). Ten patients would be incorrectly diagnosed as not having a bladder rupture (false negative), 710 would be correctly diagnosed as not having a bladder rupture (true negative), and no patients would be incorrectly classified as having a bladder rupture (false positive) (Figure, Supplemental Digital Content 3, http://links.lww.com/TA/B242).
Grading the Evidence
The Quality Assessment of Diagnostic Accuracy Studies 2 evaluation form was used to assess the quality of the diagnostic accuracy study, and risk of bias for the studies was determined to be high.[5] This was because the reference standard test (intraoperative bladder evaluation) was not always interpreted without knowledge of the index test (CT cystography). In addition, not all patients received the reference standard. The imprecision of the studies was rated as very serious due to the small sample sizes in the included studies. These factors led to the downgrading of the overall certainty of evidence to be very low (Fig. 3).
Recommendations
Based on the evidence, the panel makes the following three recommendations based on three groups of patients with different baseline risks.
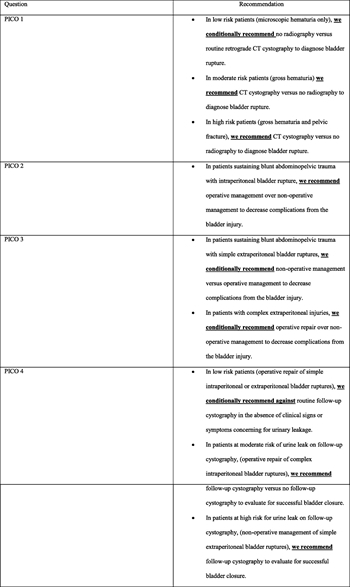
- 1A: In low-risk patients (microscopic hematuria only), we conditionally recommend no radiography versus routine retrograde CT cystography to diagnose bladder rupture (conditional recommendation based on very low-quality evidence).
The panel judged that the low likelihood of bladder ruptures in this group did not warrant the additional radiation exposure and cost associated with performing CT cystography. The 95% CI for the false positives in this group ranges from 0 to 5, meaning that up to 5 patients per 1,000 could possibly be falsely diagnosed with a bladder rupture, which may result in an unnecessary operation.
- 1B: In moderate-risk patients (gross hematuria), we recommend CT cystography versus no radiography to diagnose bladder rupture (strong recommendation for based on very low-quality evidence).
The panel makes this recommendation based on the higher likelihood of bladder rupture. Two hundred six of 1,000 patients would be diagnosed with a true bladder injury in the moderate-risk group, which would place many patients at risk for potential complications that can result from an undiagnosed bladder injury if imaging is not performed. The downside to imaging is minimal as 0 per 1,000 patients would be incorrectly diagnosed with a bladder injury and only 7 per 1,000 would be falsely diagnosed as not having a bladder injury.
- 1C: In high-risk patients (gross hematuria and pelvic fracture), we recommend CT cystography versus no radiography to diagnose bladder rupture (strong recommendation based on very low-quality evidence).
The panel makes this recommendation based on the higher likelihood of bladder rupture. Two hundred eighty of 1,000 patients would be diagnosed with bladder injury in the high-risk group, which would place many patients at risk for potential complications that can result from an undiagnosed bladder injury if imaging is not performed. The downside to imaging is minimal as 0 per 1,000 patients would be incorrectly diagnosed with a bladder injury and only 10 per 1,000 would be falsely diagnosed as not having a bladder injury.
Computed tomography cystography has the same sensitivity and specificity at detecting bladder injury as the criterion standard plain film cystography. The committee recommends that the clinician may choose either imaging modality to diagnose bladder injury based on patient condition, imaging requirements for other associated injuries, and equipment availability. Interpreting CT cystography could be less affected by overlying bone fragments caused by pelvic fracture, spine boards, or military antishock trousers that may be present on the patient in the initial evaluation of the trauma patient.
Results for Operative versus Nonoperative Management of Intraperitoneal Bladder Rupture (PICO 2)
Qualitative Synthesis
Current practice patterns for the management of intraperitoneal bladder rupture after blunt abdominal trauma involve operative repair of the injury to prevent extravasation of urine into the peritoneal cavity that can lead to peritonitis, elevated serum creatinine, azotemia, and death.[12–14] While there are very small case series describing successful nonoperative management of small intraperitoneal bladder ruptures,[15][16] the majority of the body of evidence advocates for repair of intraperitoneal bladder injuries to prevent complications related to the injury.
There were no direct comparative studies addressing PICO 2. However, two retrospective cohort studies were identified, which contained a subset of patients who underwent surgical repair and had reported outcomes.[17][18]Neither study reported on the critical outcomes of overall survival or infectious complications. Data reported for urine leak rates on the first follow-up cystogram were used as a surrogate for the critical outcome of successful bladder closure and were pooled to determine overall success rates. Significant limitations to the data existed because of lack of direct comparison groups, heterogeneity of the populations, nonstandardized study designs, and incomplete reporting of complications. In the study by Corriere and Sandler,[17] 34 of 34 patients with intraperitoneal bladder rupture who underwent surgical repair had successful bladder closure at the time of the first follow-up cystogram. In Inaba et al.,[18] 30 of 30 patients with intraperitoneal rupture who underwent surgical bladder repair had successful bladder repair at the time of the follow-up cystogram. Thus, when an intraperitoneal bladder rupture was surgically repaired, there was a 100% successful bladder repair rate.
Regarding nonoperative management of intraperitoneal bladder injuries, four case reports that contained seven patients combined were identified.[15][16][19][20] All of the isolated intraperitoneal bladder injuries were managed successfully with catheter drainage.
While both operative and nonoperative management strategies appear to have very high success rates, patients with bladder rupture can have high rates of morbidity. This has led to the current standard of practice to repair intraperitoneal bladder injuries to prevent the complications that can lead to mortality.[21][22]
Quantitative Synthesis
Meta-analysis was not possible because of lack of direct comparison between groups, heterogeneity of the populations, nonstandardized study designs, and incomplete reporting of complications among the articles.
Grading the Evidence
The data addressing PICO 2 were derived from retrospective and observational studies that provided low-quality evidence.[4] Significant limitations to the data existed because of lack of direct comparison groups, heterogeneity of the populations, nonstandardized study designs, and incomplete reporting of complications. In addition, the imprecision of the studies was rated very serious because of the small sample sizes in the included studies. These factors led to the downgrading of the overall certainty of evidence to be very low.
Recommendations
- 2: In patients sustaining blunt abdominopelvic trauma with intraperitoneal bladder rupture, we recommend operative management over nonoperative management to decrease complications from the bladder injury(strong recommendation based on very low-quality evidence).
Despite the overall certainty of evidence being very low, this recommendation is based on the panel members' judgment that the benefits of treatment clearly outweigh the harms, patient values and preferences are well understood and are largely consistent (i.e., most patients would likely choose to undergo operative repair to prevent complications such as peritonitis, fistula formation, and infectious complications).
Results for Operative versus Nonoperative Management of Extraperitoneal Bladder Rupture (PICO 3)
Qualitative Synthesis
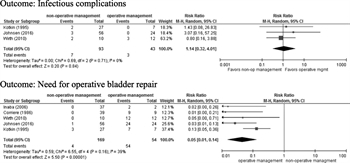
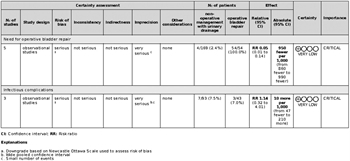
Five retrospective studies fulfilled criteria for PICO 3.[18][23–26] None of the included studies differentiated between simple and complex extraperitoneal bladder ruptures. Simple extraperitoneal bladder ruptures are defined as a single, full-thickness tear in the bladder wall resulting in spillage of urine into the extraperitoneal space, and any other concomitant bladder injury would be classified as a complex extraperitoneal bladder rupture. Wirth et al.[23] was the only article that reported any infectious complications for the operative repair group. In the pooled group of patients across all studies, there were three patients in the operative group that developed a perivesical abscess after surgical repair of the bladder. In the pooled nonoperative intervention group, four patients developed sepsis secondary to urinary tract infections, one patient developed a perivesical abscess, one patient developed infected pelvic hardware, and one patient developed osteomyelitis of the pubis.
Nonoperative management of bladder injuries resulted in a low rate (2.4%) of ultimate operative intervention to repair the bladder. The harm in the operative group was the need for the operative intervention. Therefore, the vast majority of patients with a simple extraperitoneal bladder rupture may be able to avoid an operation to repair the bladder. In contrast, current guidelines on the management of complex extraperitoneal bladder injuries from the American Urological Association recommend that these injuries be surgically repaired.[27] This recommendation was derived from the observation from case series demonstrating that complications (i.e., persistent bladder leak, abscess formation, fistula formation) from extraperitoneal bladder injuries that were managed nonoperatively were present in patients with injuries characterized as complex (i.e., bone spicules protruding into bladder lumen, concomitant rectal or vaginal lacerations, or injuries involving the bladder neck).[18][24][28–30]
Quantitative Synthesis
Five retrospective studies met the criteria for quantitative analysis, and the critical outcomes were analyzed (Fig. 4).[18][23–26] No studies reported on the critical outcome of overall survival. There was no difference in infectious complications between groups (risk ratio, 1.14 [95% CI, 0.32–4.0; p = 0.84]). The ultimate need for operative intervention to repair the bladder was significantly lower in the nonoperative intervention group (risk ratio, 0.05 [95% CI, 0.01–0.14; p < 0.00001]). Heterogeneity was minimal in the analysis for infectious complications (I [2] = 0%) and moderate in the analysis for the ultimate need for operative intervention (I [2] = 39%).
Grading the Evidence
All of the data were from small retrospective case series and observational studies. Significant limitations to the data existed because of lack of direct comparison groups, heterogeneity of the populations, nonstandardized study designs, and incomplete reporting of complications. Because of the small sample sizes and event rates in the included studies, the imprecision of the studies was rated very serious. These factors led to the downgrading of the overall certainty of evidence to be very low (Fig. 5).
Recommendations
- 3A: In patients sustaining blunt abdominopelvic trauma with simple extraperitoneal bladder ruptures, we conditionally recommend nonoperative management versus operative management to decrease complications from the bladder injury (conditional recommendation against based on very low-quality evidence).
Despite the overall certainty of evidence being very low, the panel members based this recommendation on their judgment that most patients would choose to avoid the possible complications associated with surgical repair if there was a high likelihood of spontaneous bladder healing. Only 2.4% of patients managed nonoperatively ultimately required an operation to repair the bladder. However, if in the setting of orthopedic repair of the pelvis, the bladder is to be exposed, the surgeon may consider repairing the bladder injury in the same setting to minimize exposure of the orthopedic hardware to urine.
- 3B: In patients with complex extraperitoneal injuries, we conditionally recommend operative repair over nonoperative management to decrease complications from the bladder injury (conditional recommendation based on very low-quality evidence).
The panel members made this recommendation based on their judgment that most patients would choose to avoid the more severe complications that can result from a persistent bladder leak from these complex injuries. This recommendation is consistent with recommendations from the American Urological Association to repair complex extraperitoneal bladder injuries to avoid prolonged sequelae from the injury.
Results for Cystography versus No Imaging Study after Bladder Repair (PICO 4)
In patients who have undergone operative or nonoperative management of bladder rupture (P), should cystography (I) versus no imaging study (C) be used to evaluate for bladder closure?
Qualitative Synthesis
The results of the initial follow-up cystogram were evaluated to determine the success of intervention for management of bladder ruptures. The presence or absence of a urine leak was the indicator of the success of bladder closure. Six retrospective studies were used to determine the incidence of urine leakage rates on initial follow-up imaging in low (simple extraperitoneal or intraperitoneal ruptures that are surgically repaired), medium (complex intraperitoneal ruptures that are surgically repaired), and high (simple extraperitoneal bladder rupture managed by catheter drainage) likelihood patient groups.[17][18][24–26][31] Simple intraperitoneal injuries are defined as a single full-thickness tear in the bladder wall resulting in spillage of urine into the intraperitoneal space, and any additional concomitant bladder injury would be classified as a complex intraperitoneal bladder injury. The pooled sensitivity and specificity for CT cystography from PICO 1 were then applied to each of these groups to determine the utility of retrograde CT cystography in detecting.
There was no standard time frame when the first follow-up cystogram was performed across studies. Time to first cystogram ranged from a mean of 8.6 days[31] to 10 to 14 days postinjury.[17][24][26]
Quantitative Synthesis
The critical outcomes were the true positives (patients with urine leak), false negatives (patients incorrectly classified as having urine leak), true negatives (patients without urine leak), and false positives (patients incorrectly classified as having urine leak).
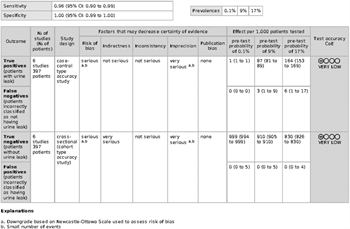
For the simple extraperitoneal or intraperitoneal ruptures that are surgically repaired (low likelihood of leak on first follow-up imaging), 0 of 175 patients demonstrated a urine leak on the first follow-up imaging study. When the pooled sensitivity and specificity of CT cystography are applied to the low likelihood (0.1%) of urine leakage group, 1 person of 1,000 would be correctly diagnosed as having a urine leak (true positive). No patients would be diagnosed incorrectly as not having a urine leak (false negative), and 999 of 1,000 would be correctly diagnosed as not having a urine leak (true negative). No patients would be classified incorrectly as having a urine leak (false positive) (Figure, Supplemental Digital Content 4, http://links.lww.com/TA/B243). Although no urine leaks were observed in the group on systematic review of the literature, the prevalence rate of 0.1% was used for calculation purposes in the evidence profile table.
For complex intraperitoneal ruptures that are surgically repaired (moderate likelihood of leak on first follow-up imaging), 2 of 22 patients demonstrated a urine leak on the first follow-up imaging study. When the pooled sensitivity and specificity of CT cystography are applied to the moderate likelihood (9%) of urine leakage group (1,000 people), 87 would be diagnosed correctly as having a urine leak (true positive); 3 would be incorrectly diagnosed as not having a urine leak (false negative); 910 would be diagnosed correctly as not having a urine leak (true negative), and no patients would be classified incorrectly as having a urine leak (false positive) (Figure, Supplemental Digital Content 5, http://links.lww.com/TA/B244).
For simple extraperitoneal ruptures that are managed nonoperatively with catheter drainage (high likelihood of leak on first follow-up imaging), 34 of 200 patients demonstrated a urine leak on the first follow-up imaging study. When the pooled sensitivity and specificity of CT cystography are applied to the group of patients with a high likelihood (17%) of urine leakage (1,000 patients), 164 would be correctly diagnosed as having a urine leak (true positive); 6 would be incorrectly diagnosed as not having a urine leak (false negative); 830 would be correctly diagnosed as not having a urine leak (true negative), and there would be no patients incorrectly classified as having a urine leak (false positive) (Figure, Supplemental Digital Content 6, http://links.lww.com/TA/B245).
Grading the Evidence
All included studies were small, retrospective, and observational. The imprecision of the studies was rated very serious because of the small sample sizes and event rates in the included studies. These factors led to the downgrading of the overall certainty of evidence to be very low (Fig. 6).
Recommendations
- 4A: In low-risk patients (operative repair of simple intraperitoneal or extraperitoneal bladder ruptures), we conditionally recommend against routine follow-up cystography in the absence of clinical signs or symptoms concerning for urinary leakage (conditional recommendation against based on very low-quality evidence).
The panel makes this recommendation based on the very low prevalence of urine leak in this group. In this risk group, 999 of 1,000 patients would unnecessarily undergo a follow-up cystogram to correctly diagnose one bladder leak.
- 4B: In patients at moderate risk of urine leak on follow-up cystography (operative repair of complex intraperitoneal bladder ruptures), we recommend follow-up cystography versus no follow-up cystography to evaluate for successful bladder closure (strong recommendation based on very low-quality evidence).
In this risk group, routine follow-up cystography in 1,000 patients would correctly diagnose 87 of 90 urinary bladder leaks with no false positive results but 3 false negatives. A total of 910 patients would undergo imaging unnecessarily. The panel judged that follow-up cystography would likely result in a large net benefit by averting complications of undiagnosed postoperative leakage.
- 4C: In patients at high risk for urine leak on follow-up cystography (nonoperative management of simple extraperitoneal bladder ruptures), we recommend follow-up cystography to evaluate for successful bladder closure (strong recommendation based on very low-quality evidence).
In this risk group, routine follow-up cystography in 1,000 patients would correctly diagnose 164 urinary bladder leaks with no false positive results but 6 false negatives. A total of 830 patients would undergo imaging unnecessarily. The panel judged that follow-up cystography would likely result in a large net benefit by averting complications of undiagnosed postoperative leakage.
Computed tomography cystography has the same sensitivity and specificity at detecting bladder injury as the criterion standard plain film cystography. The clinician may consider standard pain film cystography over CT cystography to diagnose urine leak on follow-up imaging because of decreased costs and radiation exposure.
Using These Guidelines in Clinical Practice
These evidence-based guidelines were developed after a thorough review of the literature regarding the evaluation and management of bladder injuries resulting from blunt abdominoperineal trauma. The available evidence is of very low quality, indicating that we are uncertain of the findings of the underlying report; future research is likely to change the reported estimates of effect. The GRADE approach provided a transparent process for the qualitative and quantitative of the body of evidence. These guidelines are intended to provide information to use in the decision-making process and should not replace clinical judgment.
Conclusion
Using the GRADE process, the panel makes nine recommendations based on 4 PICO questions concerning the evaluation and management of blunt force bladder injuries (Fig. 7). Several recommendations are made. The need for initial CT cystography after trauma depends on characteristics of the trauma itself but is not recommended in patients without gross hematuria. In general, patients with intraperitoneal bladder ruptures should undergo operative repair, while this is not routinely necessary in those with extraperitoneal ruptures, unless the injury is complex. The need for follow-up cystography after bladder repair depends on the risk of urine leak, with those having a low risk of urine leak not requiring a follow-up study.
Authorship
L.L.Y. and P.D. conceived the study. L.L.Y. created the PICO questions. All listed authors assisted with finalizing the PICO questions and voted on the outcomes of interest for these PICO questions. L.L.Y. and M.A.P. performed the entire literature search. L.L.Y., P.D., B.R., J.K.L., A.A.M., J.K., and J.J.C. read the abstracts and selected the articles for review. L.L.Y. reviewed and summarized the selected articles. L.L.Y. and P.D. extracted the data from the selected articles. L.L.Y. and P.D. entered the extracted data into the RevMan and GRADEpro programs and evaluated the results for recommendations. L.L.Y. wrote the article. All authors participated in the critical review of all versions of this article.
Acknowledgment
We thank librarian Mary Edwards of the Health Science Centers Library at the University of Florida for the assistance in identifying all of the literature used to develop this practice management guideline.
Disclosure
The authors declare no conflicts of interest.
References
- Carroll PR, McAninch JW. Major bladder trauma: mechanisms of injury and a unified method of diagnosis and repair. J Urol. 1984;132(2):254–257.
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–382.
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490.
- Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis Web site. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Updated 2011. Accessed January 12, 2018.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM, Group Q. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536.
- Deck AJ, Shaves S, Talner L, Porter JR. Computerized tomography cystography for the diagnosis of traumatic bladder rupture. J Urol. 2000;164(1):43–46.
- Quagliano PV, Delair SM, Malhotra AK. Diagnosis of blunt bladder injury: a prospective comparative study of computed tomography cystography and conventional retrograde cystography. J Trauma. 2006;61(2):410–421. discussion 421–22.
- Peng MY, Parisky YR, Cornwell EE 3rd, Radin R, Bragin S. CT cystography versus conventional cystography in evaluation of bladder injury. AJR Am J Roentgenol. 1999;173(5):1269–1272.
- Chan DP, Abujudeh HH, Cushing GL Jr., Novelline RA. CT cystography with multiplanar reformation for suspected bladder rupture: experience in 234 cases. AJR Am J Roentgenol. 2006;187(5):1296–1302.
- Morey AF, Iverson AJ, Swan A, Harmon WJ, Spore SS, Bhayani S, Brandes SB. Bladder rupture after blunt trauma: guidelines for diagnostic imaging. J Trauma. 2001;51(4):683–686.
- Brewer ME, Wilmoth RJ, Enderson BL, Daley BJ. Prospective comparison of microscopic and gross hematuria as predictors of bladder injury in blunt trauma. Urology. 2007;69(6):1086–1089.
- Kato A, Yoshida K, Tsuru N, Ushiyama T, Suzuki K, Ozono S, Hishida A. Spontaneous rupture of the urinary bladder presenting as oliguric acute renal failure. Intern Med. 2006;45(13):815–818.
- Merguerian PA, Erturk E, Hulbert WC Jr., Davis RS, May A, Cockett AT. Peritonitis and abdominal free air due to intraperitoneal bladder perforation associated with indwelling urethral catheter drainage. J Urol. 1985;134(4):747–750.
- Corriere JN Jr., Sandler CM. Bladder rupture from external trauma: diagnosis and management. World J Urol. 1999;17(2):84–89.
- Craggs B, Michielsen D. Conservative treatment of an intraperitoneal bladder perforation. Cent European J Urol. 2011;64(1):47–49.
- Osman Y, El-Tabey N, Mohsen T, El-Sherbiny M. Nonoperative treatment of isolated posttraumatic intraperitoneal bladder rupture in children—is it justified? J Urol. 2005;173(3):955–957.
- Corriere JN Jr., Sandler CM. Management of the ruptured bladder: seven years of experience with 111 cases. J Trauma. 1986;26(9):830–833.
- Inaba K, McKenney M, Munera F, de Moya M, Lopez PP, Schulman CI, Habib FA. Cystogram follow-up in the management of traumatic bladder disruption. J Trauma. 2006;60(1):23–28.
- Jones AL, Armitage JN, Kastner C. Conservatively managed spontaneous intraperitoneal bladder perforation in a patient with chronic bladder outflow obstruction. Urol Ann. 2014;6(4):370–372.
- Richardson JR Jr., Leadbetter GW Jr. Non-operative treatment of the ruptured bladder. J Urol. 1975;114(2):213–216.
- Matlock KA, Tyroch AH, Kronfol ZN, McLean SF, Pirela-Cruz MA. Blunt traumatic bladder rupture: a 10-year perspective. Am Surg. 2013;79(6):589–593.
- Tan LB, Chiang CP, Huang CH, Chou YH, Wang CJ. Surgical treatment of the ruptured bladder: 22 years reviewed. J Formos Med Assoc. 1990;89(11):986–991.
- Wirth GJ, Peter R, Poletti PA, Iselin CE. Advances in the management of blunt traumatic bladder rupture: experience with 36 cases. BJU Int. 2010;106(9):1344–1349.
- Corriere JN Jr, Sandler CM. Mechanisms of injury, patterns of extravasation and management of extraperitoneal bladder rupture due to blunt trauma. J Urol. 1988;139(1):43–4.
- Johnsen NV, Young JB, Reynolds WS, Kaufman MR, Milam DF, Guillamondegui OD, Dmochowski RR. Evaluating the role of operative repair of extraperitoneal bladder rupture following blunt pelvic trauma. J Urol. 2016;195(3):661–665.
- Kotkin L, Koch MO. Morbidity associated with nonoperative management of extraperitoneal bladder injuries. J Trauma. 1995;38(6):895–898.
- Morey AF, Brandes S, Dugi DD 3rd, Armstrong JH, Breyer BN, Broghammer JA, Erickson BA, Holzbeierlein J, Hudak SJ, Pruitt JH, et al. Urotrauma: AUA guideline. J Urol. 2014;192(2):327–335.
- Cass AS, Johnson CF, Khan AU, Matsuura JK, Godec CJ. Nonoperative management of bladder rupture from external trauma. Urology. 1983;22(1):27–29.
- Cass AS, Luxenberg M. Features of 164 bladder ruptures. J Urol. 1987;138(4):743–745.
- Urry RJ, Clarke DL, Bruce JL, Laing GL. The incidence, spectrum and outcomes of traumatic bladder injuries within the Pietermaritzburg Metropolitan Trauma Service. Injury. 2016;47(5):1057–1063.
- Inaba K, Okoye OT, Browder T, Best C, Branco BC, Teixeira PG, Barmparas G, Reddy S, Demetriades D. Prospective evaluation of the utility of routine postoperative cystogram after traumatic bladder injury. J Trauma Acute Care Surg. 2013;75(6):1019–1023.
Keywords:
Genitourinary trauma; bladder injury; hematuria; cystography