Clostridium Difficile-Associated Disease - Timing and Type of Surgical Treatment
Published 2014
Citation: J Trauma. 76(6):1484-1493, June 2014.
Authors
Ferrada, Paula MD; Velopulos, Catherine G. MD, MHS; Sultan, Shahnaz MD; Haut, Elliott R. MD; Johnson, Emily MLIS; Praba-Egge, Anita MD, PhD; Enniss, Toby MD; Dorion, Heath MD; Martin, Niels D. MD; Bosarge, Patrick MD; Rushing, Amy MD; Duane, Therese M. MD
Author Information
From the Department of Surgery (P.F., E.J., T.M.D.), Virginia Commonwealth University Medical Center, Medical College of Virginia, Richmond, Virginia; Department of Surgery and Center for Surgical Trials and Outcomes Research (CSTOR) (C.G.V., E.R.H.), The Johns Hopkins School of Medicine, Baltimore, Maryland; Malcom Randall VAMC, Division of Gastroenterology, Hepatology, and Nutrition (S.S.), University of Florida College of Medicine, Gainesville, Florida; Department of Surgery (A.P.-E.), Maine General Health, Oakland, Maine; Department of Surgery (T.E.), University of Utah School of Medicine, Salt Lake City, Utah; Department of Surgery (H.D.), Northeast Ohio Medical University, Rootstown, Ohio; Department of Surgery (N.D.M.), University of Pennsylvania, Philadelphia; Department of Surgery (A.R.), York Hospital, York, Pennsylvania; and Department of Surgery (P.B.), University of Alabama—Birmingham, Birmingham, Alabama.
Submitted: January 15, 2014, Revised: February 21, 2014, Accepted: February 25, 2014.
P.F. and C.G.V. contributed equally to this work and should be noted as co–first authors.
Address for reprints: Paula Ferrada, MD, VCU Surgery, Trauma, Critical Care and Emergency Surgery, West Hospital, 15th Floor, East Wing1200 E Broad St, Richmond, VA 23298, PO Box 980454, Richmond, VA 23298-0454; email: pferrada@mcvh-vcu.edu.
Objectives
The objective of this guideline was to evaluate whether surgical timing (early vs. late) and type (total abdominal colectomy [TAC] vs. other surgical options) are associated with better outcomes in patients with severe CDAD.
The population (P), intervention (I), comparator (C), and outcome (O) questions are defined as follows:
- PICO Question 1: In adult patients with CDAD, does early surgery compared with late surgery (as defined by the need for vasopressors) decrease mortality rates?
- PICO Question 2: In adult patients with CDAD, does the use of TAC compared with other types of surgical interventions decrease mortality rates?
Inclusion Criteria For This Review
Study Types
For the purpose of making recommendations, studies included randomized controlled trials, prospective observational or retrospective studies, and case control studies. Meta-analyses, case reports, letters, and reviews containing no original data or comments were excluded.
Participant Types
We included studies of adult patients without restricting sex, ethnicity, or degree of comorbidity. Only studies pertaining to the treatment of hospitalized patients with CDAD were included. CDAD was defined as severe CDI resulting in clinical deterioration, such as multiorgan system failure, peritonitis, and/or sepsis as a consequence of the disease.
Intervention Type
We included studies in which TAC or subtotal colectomy (each defined as removal of most of the colon excluding the rectum) was performed compared with other procedures such as segmental colectomy, exploratory laparotomy without colectomy, or ostomy formation.
Outcome Measure Types
Outcomes were chosen by the team and rated in importance from 1 to 9, with scores of 7 to 9 representing critical outcomes. The following outcomes were considered by the committee members: length of stay, ICU length of stay, cost, ventilator-free days, renal failure, and respiratory failure. However, all of these criteria were deemed noncritical for the decision-making process within the GRADE framework. In addition, the available literature did not provide sufficient or consistent measurements across the studies, specifically if the onset of related conditions such as renal or respiratory failure occurred before or after surgical intervention. Only a reduction in mortality was deemed a critical outcome for the decision-making process, and this was chosen as the primary outcome measure.
Review Methods
Search Strategy
With the assistance of an information specialist, we conducted a systematic search of the , EMBASE, and Cochrane Library databases for studies published from 1992 to January 2014. The search used the following MeSH terms alone or in combination: Clostridium difficile, colitis, colectomy, surgery, and mortality. We used the “Related Articles” function to broaden the search and scan all citations for relevance. We used only articles available in English. In addition to the electronic search, we manually searched the bibliographies of recent reviews and articles.
Study Selection
After completing the literature search, two independent reviewers screened the titles and abstracts; any disagreement on inclusion was resolved through consensus. We excluded case reports and narrative review articles. The resulting studies were subjected to full-text review by two independent reviewers.
Data Extraction and Management
Using a form developed by the team, two independent reviewers extracted data from the individual studies into Microsoft Excel, using double data entry for accuracy. We then entered these data into Review Manager X.6 (Review Manager [RevMan] [Computer program], Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012), including information on the authors, study number, country of origin, study methodology, population, intervention, and relevant outcome measures.
Methodological Quality Assessment
The articles were evaluated using the GRADE framework,[12][14–29] which describes four levels of quality of evidence: high, moderate, low, and very low. Quality of evidence is reflected as the extent to which one can be confident that an estimate of effect is correct and includes an explicit consideration of the following domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias.[21–26] The data were entered into GRADEpro for the generation of evidence tables.
Recommendations are based on the overall quality of evidence with implicit consideration of the risk-benefit ratio and patients’ values and preferences. Strong recommendations are prefaced by the statement “we strongly recommend,” while weak recommendations are prefaced by the statement “we suggest” or “we conditionally recommend” as per the GRADE methodology.
Measures of Treatment Effect
We reported the dichotomous outcome of mortality as a risk ratio (RR) with associated 95% confidence intervals (CIs) and p values since the baseline incidence of the primary outcome was thought to be relatively high in this population (>20%). The unit of analysis was individual patients.
Assessment of Heterogeneity
Potential heterogeneity exists because of population differences, different types of surgery performed, and how patients are defined. We examined these differences across studies to assess the clinical and methodological heterogeneity. For the meta-analysis, we used RevMan to calculate the Q statistic, and then the I[2] statistic (%) was used to determine the proportion of variation between studies attributable to heterogeneity and categorized as “low” (25–49%), “moderate” (50–74%), or “high” (74–100%). We also used the χ[2] test for heterogeneity and examined the CIs for overlap, with decreasing overlap representing increasing heterogeneity.
Data Synthesis (Meta-analysis)
We performed a meta-analysis of the outcome of mortality rate for each PICO question by using the RevMan software. We used the DerSimonian and Laird random-effects model method[30] because our studies did not share a common effect size and unknown influential factors could vary across studies (unknown confounders). This allowed us to incorporate both the intrastudy and interstudy variability along a distribution of the “true” effects, which weighs larger and smaller studies more evenly. Potential heterogeneity across studies was assessed using the Q statistic, I[2] statistic (%), and χ[2] test for heterogeneity. If heterogeneity was “moderate” to “high,” we did not consider pooling the data; rather, we performed a qualitative narrative summary of the results only.
Sensitivity Analysis
We conducted a sensitivity analysis for PICO Question 2 to investigate the implications of the ultimate surgery type performed compared with the first surgery since some patients underwent more than one procedure. A further analysis was performed to examine only those studies that reported their conversion rates from other procedures to TAC.
Results
The original search yielded 62 studies; after the elimination of studies that did not contain the original data, only 38 were deemed appropriate for full-text review. We further excluded six studies: one excluded study included a pediatric population, another was descriptive in nature, and four did not address the specific questions outlined in our review. We ultimately included 32 studies in this guideline for recommendations.[3–5][9–11][31–56] Of these articles, there were no randomized trials; two were prospective studies,[4][5][11][31–37][56] while the remaining were retrospective (Table 1). Note that in this table, some studies included the total number of patients with CDI as well as those patients who had CDAD that required intervention, while others included only patients with CDAD. As outlined in the PRISMA diagram in Figure 1, we included all 32 studies in the qualitative synthesis. We then identified eight studies that were appropriate for quantitative synthesis (meta-analysis) for PICO Question 1, and 17 studies for PICO Question 2.
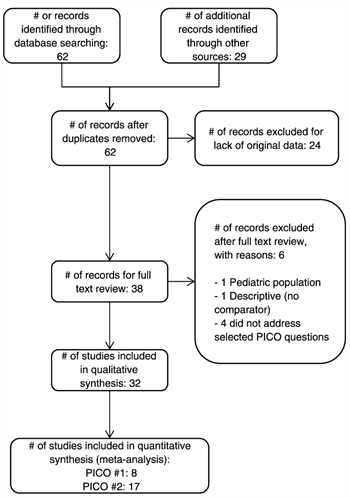
Figure 1. PRISMA flow diagram for study selection.
Results Obtained For Pico Question 1
In adult patients with CDAD (P), does early surgery (I) compared with late surgery (C) (as defined by the need for vasopressors) decrease mortality rates (O)?
Qualitative Synthesis
Overall surgical mortality was 19% to 80% in the included studies (Table 1). Seder et al.[54] described 6,841 patients with CDAD and showed a decreased mortality associated with surgery before the development of the vasopressor requirement. The experience of Hall et al.[38] with 3,237 consecutive cases of CDAD showed an increased mortality rate when surgical exploration was performed after intubation or the development of respiratory failure and the use of vasopressors.
Longo et al.[47] found that peritonitis and bowel perforation indicative of delayed surgical therapy were associated with increased mortality rates. Delaying surgery until after the development of respiratory failure, defined as unplanned intubation, was found to result in increased mortality rates in six articles,[4][9][38][44][54][57] while similar findings were noted in four studies when surgical intervention followed renal failure.[38][44][54][57] Finally, in 10 studies, delaying surgery until the patient became hemodynamically unstable and required vasopressors was associated with increased mortality rates.[4][9][10][32][38][39][44][48][52][54][57] Overall, these studies suggested that early surgery before the development of shock or organ failure was associated with lower mortality rates in patients with CDAD.
While most studies do not address a specific period in which surgery should be considered, a few studies suggest that the window for optimal surgical management is relatively short. In the study of Ali et al.,[32] survivors had surgery at a mean of 3.2 days, compared with nonsurvivors at 5.4 days. Sailhamer et al.[9] similarly showed that of patients admitted to the ICU for CDAD, mean time to the operating room for survivors was 1.9 days compared with a mean time of 3.9 days for nonsurvivors. The most recent article addressing the association with mortality and specific timing of surgery in patients with CDAD was published in November 2013.[57] Halabi et al.[57] retrospectively reviewed the Nationwide Inpatient Sample from 2001 to 2010 for colectomy in patients with C. difficile colitis and the association with mortality. The overall mortality associated with colectomy was 30.7%. Surgical intervention delayed for more than 3 days after admission in patients with CDAD was associated with higher mortality rates (odds ratio, 1.09; 95% CI, 1.05–1.14; p < 0.05).
Quantitative Synthesis (Meta-analysis)
Before pooling study data, we assessed methodological and clinical heterogeneity across the studies and found variability in how the intervention and comparator were defined. The most commonly defined measure of early versus late surgery was progression to the need for vasopressors. With the use of this definition, eight studies were included in the meta-analysis. We found that early surgery was associated with reduced mortality rates with an RR of 0.50 (95% CI, 0.35–0.72; Fig. 2). Of note, the I[2] statistic was 39%, falling into the “low” heterogeneity category, indicating that the studies were comparable.
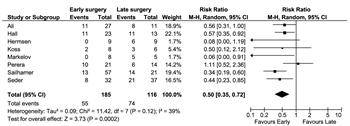
Figure 2. Early versus late surgery defined as need for vasopressors, meta-analysis.
Grading the Evidence
With the use of the GRADE framework for assessing the outcome of reduced mortality rates, no serious risk of bias, inconsistency, indirectness, or publication bias was found. However, severe imprecision was noted since the studies were small and the CIs were large. Starting from observational studies (which are considered low quality), we then rated down for imprecision. Therefore, the overall quality of evidence was very low (see the GRADE profile shown in Fig. 3).
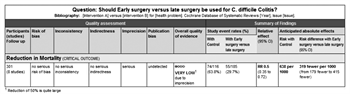
Figure 3. Early versus late surgery defined as need for vasopressors, evidence profile.
Discussion
Early surgery for patients with fulminant CDAD is associated with reduced mortality rates. In the absence of validated prediction scores, determining which CDAD patients will progress to shock can be difficult to elucidate. However, the early signs of hemodynamic stability, such as low mean arterial pressures or decreasing urine output, can potentially serve as triggers for the need for surgical intervention (early surgery) before the development of multiorgan system failure or shock.
Using hemodynamic instability as the trigger for surgical intervention is associated with increased mortality, and patients should proceed to surgery before progression to shock. Although an exact time frame is unclear, the current data suggest that this is usually within 3 days to 5 days after diagnosis if patients are worsening or not clinically improving. While this is not a definite time frame, it may be helpful to guide clinicians in identifying patients prospectively who are at risk for proceeding to hemodynamic instability. We anticipate that this will be enhanced in the future as a physiologic scoring system is refined.[8]
Recommendation
Within the GRADE framework, once the overall quality of evidence across studies and outcomes is determined, the guideline panel will make a recommendation that considers the following: quality of the body of evidence, patients’ values and preferences, and cost/resource use. Despite the overall quality of evidence being very low, the panel considered that most patients would place a high value on the potential 50% reduction in mortality and that the potential benefit outweighs any potential harm in performing surgery early. This allows for a strong recommendation. Within the GRADE framework, a strong recommendation implies that most individuals would want the recommended course of action and only a small proportion would not.[12–14]
RECOMMENDATION: In adult patients with CDAD, we strongly recommend that patients undergo surgery early, that is, before the development of shock or the need for vasopressors. This recommendation is based on very low quality evidence but considers that individual patients will place a high value on the overall benefit (reduced mortality rates).
Results For Pico Question 2
In adult patients with CDAD (P), does TAC (I) compared with other types of surgical intervention (C) decrease mortality rates (O)?
Qualitative Synthesis
In some studies,[4][5][31][32][37][38][41][43][47][48][52] total colectomy carried a higher mortality rate than partial colectomy, although this was not statistically significant. In these cases, it was not specified whether patients who underwent total colectomy were sicker or had extensive necrosis and tissue damage as an indication for total rather than partial colectomy. Several studies assigned mortality or survival to the initial procedure performed despite the fact that many patients were later converted to TAC (an intention-to-treat approach). These studies were likely confounded by timing, with later conversions to TAC representing a delay in appropriate treatment, thus assigning mortality inappropriately.
Klipfel et al.[43] showed that CDAD and an acute abdomen is a lethal combination that carries an 80% mortality rate regardless of the procedure performed; however, a major limitation of these findings was that these patients received prolonged antibiotic treatments and that the decision for surgical intervention was purely subjective. Recognizing that the diagnosis and treatment of CDAD have evolved during the past two decades, the 1994 study by Lipsett et al.[46]found that partial colectomy is uniformly fatal, with a mortality rate of 100% versus a mortality rate of 14% in patients undergoing total colectomy. In this series, a worse outcome was associated with the presence of peritonitis as shown in earlier studies.[46]
Quantitative Synthesis (Meta-analysis)
Although there was minimal methodological heterogeneity, there was some clinical heterogeneity because the comparator to TAC was not consistent across all studies. Of note, however, the number of patients undergoing procedures other than TAC or partial colectomy was quite low. The I[2] statistic was “low” at 48%, although this is likely due to the wide CIs of each study rather than a true homogeneity between the studies.
Twenty studies compared total colectomy versus other procedures or no surgery as treatment for CDAD,[4][5][10][31][32][34][35][37][38][41][43][44][46–49][52–56] 17 of which had sufficient data for analysis. Because the ultimate procedure performed likely dictates prognosis, this was chosen for the comparison group.
When considering only the first procedure performed, mortality seemed to trend higher for TAC, with an RR of 1.11 (95% CI, 0.69–1.80). When taking into account that many patients were converted to TAC in a later operation, the point estimate actually switches sides, showing a slight change in results, with an RR of 0.86 (95% CI, 0.56–1.31). This is shown in Figure 4. Although neither reaches statistical significance (likely due to power), change in direction of the point estimate is compelling within the construct of evidence-based medicine.
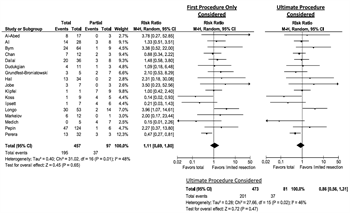
Figure 4. Total colectomy versus partial or no resection, meta-analysis.
A sensitivity analysis was performed on the subgroup of studies that reported conversion rates to investigate the potential confounding impact of the delay associated with first performing a procedure other than TAC. The ultimate procedure performed was compared with a best-case scenario where all patients who converted instead underwent TAC first and survived, compared with a worst-case scenario in which all patients who were converted underwent TAC first and died. In each case, the point estimate favored TAC, although the CIs crossed the null hypothesis (Fig. 5).
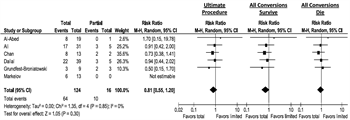
Figure 5. Subgroup analysis, total colectomy versus partial or no resection—conversion rate given; sensitivity analysis evaluating best- and worst-case scenario for conversion.
Grading the Evidence
With the use of the GRADE framework, no serious risk of bias, indirectness, or publication bias was detected; however, we rated down for inconsistency (which was apparent due to clinical heterogeneity and a borderline I[2] statistic) and imprecision (due to few events and wide CIs) (Fig. 6).

Figure 6. Ultimate procedure, total colectomy versus partial or no resection, evidence profile.
Discussion
In the worst-case scenario with the given data, total colectomy does not increase mortality. In the best-case scenario with the given data, total colectomy may decrease mortality. Many of the patients who ultimately died after total colectomy had significant delay in this procedure, with many first undergoing a more limited procedure, thus blunting the effectiveness of the total colectomy and making our positive estimate likely too conservative. In addition, in each of the studies, the proportion of patients undergoing less than a total colectomy is quite small. This likely reflects selection bias. Most of the studies suggest that the patients were quite different, with the patients undergoing total colectomy being much sicker, with having more comorbidities, thus having a higher baseline mortality.
A recent meta-analysis by Bhangu et al.[58] showed a point estimate for total colectomy that was unfavorable. Because the CIs cross the null hypothesis and indicate no difference, they could only accurately conclude that total colectomy is a reasonable option. Their ultimate conclusion (in opposition to their point estimate) that total colectomy is superior and thus the procedure of choice was interestingly not supported by their meta-analysis.
In our meta-analysis, we considered that having multiple operations constituted a treatment failure at initial operation, such that the ultimate operation performed would have significant impact. When we explored this, we did indeed find that our effect estimate shifted to the other side, in favor of total colectomy. Although the null hypothesis is still included, the magnitude of the effect is a nearly 15% reduction in mortality, and that the effect changes direction is compelling. On subgroup analysis of the studies specifically reporting conversions from limited procedures to total colectomy, we find similar estimates, with sensitivity analysis considering best- and worst-case scenarios for mortality after conversion, maintaining a point estimate in favor of total colectomy.
Recommendation
While the overall body of evidence that informs this recommendation is very low, the authors took into account that potential confounding factors (i.e., delay in undergoing TAC as the procedure) could plausibly lead to an underestimated version of the true effect. In this case, that sicker patients (having failed the initial procedure) were converted to TAC but still seemed to fare better increases our confidence in the intervention.
In addition, the panel again considered patients’ values and preferences, noting that most patients would favor definitive treatment at the time of initial surgical intervention and in weighing the desirable and undesirable outcomes, they would again place a high value on one definitive treatment that could reduce their mortality. Taking into account all these factors, the panel voted for a conditional recommendation in favor of TAC.
RECOMMENDATION: In adult patients with CDAD undergoing surgery, we conditionally recommend total or subtotal colectomy (vs. partial colectomy or other surgery). This recommendation is based on very low-quality evidence but places a high value on patient preferences for a definitive surgical intervention that may more effectively reduce mortality rates.
Future Investigation
A multi-institutional randomized controlled trial (ClinicalTrials.gov identifier NCT01441271) entitled “Optimal surgical treatment of fulminant Clostridium difficile colitis”[59] was initiated to determine the best operative procedure for patients with CDAD. In this study, patients were intended to be randomized to ileostomy with colonic lavage versus total colectomy; however, the study was recently closed due to a lack of meaningful enrollment.
Neal et al.[11] described an innovative procedure in which an ileostomy is created to allow for colonic washing and direct topical treatment with vancomycin enemas. This article quoted a 19% mortality rate in these patients compared with a 50% mortality rate in historical controls who underwent total colectomy. The article showed encouraging results; however, the recurrence rate after ileostomy reversal and a longer follow-up period need to be reported in addition to reproducibility verification in other institutes before this procedure can be accepted as a standard of care. Additional studies evaluating this treatment and other novel surgical approaches are also needed. Finally, further investigation is required to evaluate scoring systems that can predict deterioration to better inform decision making with regard to surgery timing and type.
Using These Guidelines In Clinical Practice
These guidelines represent a very detailed summary of the literature regarding CDAD and surgical timing and type and are meant to inform the decision-making process, not replace clinical judgment. Patients with fulminant CDAD carry a very high mortality rate. The literature available for review thus far supports the course of earlier intervention when deterioration is present and total colectomy as the procedure of choice.
Conclusion
In summary, two important evidence-based recommendations can be provided by using the GRADE methodology (Fig. 7). First, we strongly recommend that adult patients with CDAD undergo early surgery before developing shock and the need for vasopressors. The literature suggests that this may be between 3 days and 5 days after diagnosis in patients who are worsening or not clinically improving. Second, in adult patients with CDAD undergoing surgery, we conditionally recommend total or subtotal colectomy (vs. partial colectomy or other surgery) when the diagnosis of C. difficile colitis is known.

Figure 7. Recommendations.
Authorship
P.F., T.M.D., and E.R.H. conceived the study. P.F. created the PICO questions. P.F., C.G.V., E.R.H., A.P.-E., T.E., H.D., N.D.M., P.B., A.R., and T.M.D. voted regarding the outcomes of interest for these PICO questions. H.D. and N.D.M. evaluated the articles independently for other outcomes, specifically renal failure and respiratory failure. E.J. and P.F. performed the entire literature search, read all of the abstracts, and selected the articles for review. P.F., C.G.V., E.R.H., A.P.-E., T.E., H.D., N.D.M., P.B., A.R., and T.M.D. reviewed and summarized the selected articles. P.F. extracted the data from the selected articles. S.S. and P.F. entered the extracted data into the RevMan and GRADEpro programs and evaluated the results for recommendations. C.G.V. extracted the data and performed the same task independently. P.F. and C.G.V. wrote the manuscript. P.F. and C.G.V. contributed equally to this work and should be noted as co–first authors. S.S. reviewed the manuscript for methodological content and made critical revisions to the final draft. All authors participated in the critical review of all versions of this article.
Acknowledgment
We thank the EAST and the EAST foundation for the opportunity to write this article, for the specialized training provided to the authors regarding the GRADE method, and for the detail-oriented peer review of multiple versions of this article by the guidelines committee.
Disclosure
The authors declare no conflicts of interest.
References
- Carchman EH, Peitzman AB, Simmons RL, Zuckerbraun BS. The role of acute care surgery in the treatment of severe, complicated Clostridium difficile–associated disease. J Trauma Acute Care Surg. 2012; 73: 789–800.
- Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile–associated diarrhea with high morbidity and mortality. N Engl J Med. 2005; 353: 2442–2449.
- Kent KC, Rubin MS, Wroblewski L, Hanff PA, Silen W. The impact of Clostridium difficile on a surgical service: a prospective study of 374 patients. Ann Surg. 1998; 227: 296–301.
- Byrn JC, Maun DC, Gingold DS, Baril DT, Ozao JJ, Divino CM. Predictors of mortality after colectomy for fulminant Clostridium difficile colitis. Arch Surg. 2008; 143: 150–154.
- Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002; 235: 363–372.
- Maseda E, Hernandez-Gancedo C, Lopez-Tofino A, Suarez-de-la Rica A, Garcia-Bujalance S, Gilsanz F. Use of fidaxomicin through a nasogastric tube for the treatment of septic shock caused by Clostridium difficile infection in a patient with oral cancer admitted to the Surgical Critical Care Unit. Rev Esp Quimioter. 2013; 26: 375–377.
- van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013; 368: 407–415.
- van der Wilden GM, Chang Y, Cropano C, et al. Fulminant Clostridium difficile colitis: prospective development of a risk scoring system. J Trauma Acute Care Surg. 2014; 76: 424–430.
- Sailhamer EA, Carson K, Chang Y, et al. Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. Arch Surg. 2009; 144: 433–439.
- Perera AD, Akbari RP, Cowher MS, et al. Colectomy for fulminant Clostridium difficile colitis: predictors of mortality. Am Surg. 2010; 76: 418–421.
- Neal MD, Alverdy JC, Hall DE, Simmons RL, Zuckerbraun BS. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Ann Surg. 2011; 254: 423–427.
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011; 64: 383–394.
- Kerwin AJ, Haut ER, Burns JB, et al. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg. 2012; 73: S283–S287.
- Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013; 66: 151–157.
- Andrews JC, Schunemann HJ, Oxman AD, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol. 2013; 66: 726–735.
- Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013; 66: 719–725.
- Guyatt GH, Thorlund K, Oxman AD, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013; 66: 173–183.
- Guyatt GH, Oxman AD, Schunemann HJ. GRADE guidelines—an introduction to the 10th–13th articles in the series. J Clin Epidemiol. 2013; 66: 121–123.
- Brunetti M, Shemilt I, Pregno S, et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. J Clin Epidemiol. 2013; 66: 140–150.
- Guyatt GH, Oxman AD, Santesso N, et al. GRADE guidelines: 12. Preparing summary of findings tables—binary outcomes. J Clin Epidemiol. 2013; 66: 158–172.
- Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011; 64: 1283–1293.
- Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011; 64: 1294–1302.
- Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol. 2011; 64: 1277–1282.
- Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011; 64: 1303–1310.
- Guyatt GH, Oxman AD, Sultan S, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011; 64: 1311–1316.
- Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011; 64: 407–415.
- Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011; 64: 401–406.
- Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011; 64: 395–400.
- Guyatt GH, Oxman AD, Kunz R, et al. Going from evidence to recommendations. BMJ. 2008; 336: 1049–1051.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7: 177–188.
- Al-Abed YA, Gray EA, Rothnie ND. Outcomes of emergency colectomy for fulminant Clostridium difficile colitis. Surgeon. 2010; 8: 330–333.
- Ali SO, Welch JP, Dring RJ. Early surgical intervention for fulminant pseudomembranous colitis. Am Surg. 2008; 74: 20–26.
- Ananthakrishnan AN, McGinley EL, Saeian K, Binion DG. Temporal trends in disease outcomes related to Clostridium difficile infection in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011; 17: 976–983.
- Chan S, Kelly M, Helme S, Gossage J, Modarai B, Forshaw M. Outcomes following colectomy for Clostridium difficile colitis. Int J Surg. 2009; 7: 78–81.
- Dudukgian H, Sie E, Gonzalez-Ruiz C, Etzioni DA, Kaiser AM. C. difficile colitis—predictors of fatal outcome. J Gastrointest Surg. 2010; 14: 315–322.
- Gash K, Brown E, Pullyblank A. Emergency subtotal colectomy for fulminant Clostridium difficile colitis—is a surgical solution considered for all patients? Ann R Coll Surg Engl. 2010; 92: 56–60.
- Grundfest-Broniatowski S, Quader M, Alexander F, Walsh RM, Lavery I, Milsom J. Clostridium difficile colitis in the critically ill. Dis Colon Rectum. 1996; 39: 619–623.
- Hall JF, Berger D. Outcome of colectomy for Clostridium difficile colitis: a plea for early surgical management. Am J Surg. 2008; 196: 384–388.
- Hermsen JL, Dobrescu C, Kudsk KA. Clostridium difficile infection: a surgical disease in evolution. J Gastrointest Surg. 2008; 12: 1512–1517.
- Issa M, Vijayapal A, Graham MB, et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007; 5: 345–351.
- Jobe BA, Grasley A, Deveney KE, Deveney CW, Sheppard BC. Clostridium difficile colitis: an increasing hospital-acquired illness. Am J Surg. 1995; 169: 480–483.
- Kenneally C, Rosini JM, Skrupky LP, et al. Analysis of 30-day mortality for Clostridium difficile–associated disease in the ICU setting. Chest. 2007; 132: 418–424.
- Klipfel AA, Schein M, Fahoum B, Wise L. Acute abdomen and Clostridium difficile colitis: still a lethal combination. Dig Surg. 2000; 17: 160–163.
- Koss K, Clark MA, Sanders DS, Morton D, Keighley MR, Goh J. The outcome of surgery in fulminant Clostridium difficile colitis. Colorectal Dis. 2006; 8: 149–154.
- Kurian A, Suryadevara S, Ramaraju D, et al. In-hospital and 6-month mortality rates after open elective vs open emergent colectomy in patients older than 80 years. Dis Colon Rectum. 2011; 54: 467–471.
- Lipsett PA, Samantaray DK, Tam ML, Bartlett JG, Lillemoe KD. Pseudomembranous colitis: a surgical disease? Surgery. 1994; 116: 491–496.
- Longo WE, Mazuski JE, Virgo KS, Lee P, Bahadursingh AN, Johnson FE. Outcome after colectomy for Clostridium difficile colitis. Dis Colon Rectum. 2004; 47: 1620–1626.
- Markelov A, Livert D, Kohli H. Predictors of fatal outcome after colectomy for fulminant Clostridium difficile colitis: a 10-year experience.dr.markelov@gmail.com. Am Surg. 2011; 77: 977–980.
- Medich DS, Lee KK, Simmons RL, Grubbs PE, Yang HC, Showalter DP. Laparotomy for fulminant pseudomembranous colitis. Arch Surg. 1992; 127: 847–852.
- Morris AM, Jobe BA, Stoney M, Sheppard BC, Deveney CW, Deveney KE. Clostridium difficile colitis: an increasingly aggressive iatrogenic disease? Arch Surg. 2002; 137: 1096–1100.
- Muto CA, Pokrywka M, Shutt K, et al. A large outbreak of Clostridium difficile–associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005; 26: 273–280.
- Pepin J, Vo TT, Boutros M, et al. Risk factors for mortality following emergency colectomy for fulminant Clostridium difficile infection. Dis Colon Rectum. 2009; 52: 400–405.
- Rubin MS, Bodenstein LE, Kent KC. Severe Clostridium difficile colitis. Dis Colon Rectum. 1995; 38: 350–354.
- Seder CW, Villalba MR Jr, Robbins J, et al. Early colectomy may be associated with improved survival in fulminant Clostridium difficile colitis: an 8-year experience. Am J Surg. 2009; 197: 302–307.
- Synnott K, Mealy K, Merry C, Kyne L, Keane C, Quill R. Timing of surgery for fulminating pseudomembranous colitis. Br J Surg. 1998; 85: 229–231.
- Trudel JL, Deschenes M, Mayrand S, Barkun AN. Toxic megacolon complicating pseudomembranous enterocolitis. Dis Colon Rectum. 1995; 38: 1033–1038.
- Halabi WJ, Nguyen VQ, Carmichael JC, Pigazzi A, Stamos MJ, Mills S. Clostridium difficile colitis in the United States: a decade of trends, outcomes, risk factors for colectomy, and mortality after colectomy. J Am Coll Surg. 2013; 217: 802–812.
- Bhangu A, Nepogodiev D, Gupta A, Torrance A, Singh P. Systematic review and meta-analysis of outcomes following emergency surgery for Clostridium difficile colitis. Br J Surg. 2012; 99: 1501–1513.
- Massachusetts General Hospital. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of fulminant Clostridium difficilecolitis: a randomized controlled trial. In: ClinicalTrials.gov. Bethesda, MD: National Library of Medicine (US); 2011. Updated February 23, 2013; Available at: http://clinicaltrials.gov/show/NCT01441271NLM Identifier: NCT0144127. Accessed December 10, 2013.
Table
|
|
|
|
|
|
Initial Operation |
|
|
||
|---|---|---|---|---|---|---|---|---|---|
|
Author |
Country |
Type |
Total |
Surgery, |
TAC |
Limited rsxn or Stoma |
No resection |
Data Early vs. Late |
Overall Surgical Mortality, % |
|
Al-Abed[31] 2010 |
United Kingdom |
Retrospective |
528 |
20 |
17 |
3 |
— |
— |
40 |
|
Ali 2008 |
United States |
Retrospective |
36 |
36 |
28 |
8 |
— |
X |
47 |
|
Ananthakrishan[32] 2011 |
United States |
Retrospective |
13,713 |
574 |
574 |
— |
— |
— |
non recorded |
|
Byrn[33] 2008 |
United States |
Retrospective |
5,718 |
73 |
63 |
10 |
— |
— |
34 |
|
Chan[34] 2009 |
United Kingdom |
Retrospective |
15 |
15 |
12 |
3 |
— |
— |
67 |
|
Dallal 2002 |
United States |
Retrospective |
2,334 |
44 |
36 |
5 |
3 |
— |
57 |
|
Dudukgian[35] 2010 |
United States |
Retrospective |
398 |
14 |
11 |
2 |
1 |
— |
36 |
|
Gash[36] 2010 |
United Kingdom |
Retrospective |
1,398 |
17 |
16 |
1 |
— |
— |
53 |
|
Grundfest[37] 1996 |
United States |
Retrospective |
59 |
12 |
5 |
5 |
2 |
— |
42 |
|
Halabi[57] 2013 |
United States |
Retrospective |
562,247 |
3,900 |
3,900 |
— |
— |
X |
32 |
|
Hall[38] 2008 |
United States |
Retrospective |
3,237 |
36 |
34 |
2 |
— |
X |
36 |
|
Hermsen[39] 2008 |
United States |
Retrospective |
7,588 |
13 |
13 |
— |
— |
X |
46 |
|
Issa[40] 2007 |
United States |
Retrospective |
60 |
12 |
12 |
— |
— |
— |
NR |
|
Jobe[41] 1995 |
United States |
Retrospective |
201 |
10 |
6 |
1 |
3 |
— |
30 |
|
Kenneally[42] 2007 |
United States |
Retrospective |
278 |
6 |
— |
— |
— |
— |
33 |
|
Klipfel[43] 2000 |
United States |
Retrospective |
223 |
10 |
1 |
3 |
6 |
— |
80 |
|
Koss[44] 2006 |
United Kingdom |
Retrospective |
3,472 |
14 |
9 |
5 |
— |
X |
36 |
|
Kurian[45] 2011 |
United States |
Retrospective |
14 |
14 |
14 |
— |
— |
— |
28 |
|
Lipsett[46] 1994 |
United States |
Retrospective |
3,300 |
13 |
7 |
6 |
— |
— |
38 |
|
Longo[47] 2004 |
United States |
Retrospective |
67 |
67 |
53 |
14 |
— |
— |
48 |
|
Markelov[48] 2011 |
United States |
Retrospective |
13 |
13 |
12 |
1 |
— |
X |
46 |
|
Medich[49] 1992 |
United States |
Retrospective |
12 |
12 |
5 |
3 |
4 |
— |
33 |
|
Morris[50] 2002 |
United States |
Retrospective |
157 |
12 |
12 |
— |
— |
— |
25 |
|
Muto[51] 2005 |
United States |
Retrospective |
419 |
26 |
— |
— |
— |
— |
NR |
|
Neal[11] 2011 |
United States |
Prospective |
42 |
42 |
— |
42 |
— |
— |
19 |
|
Pepin[52] 2009 |
Canada |
Retrospective |
130 |
130 |
124 |
6 |
— |
— |
37 |
|
Perera[10] 2010 |
United States |
Retrospective |
35 |
35 |
32 |
3 |
— |
X |
46 |
|
Rubin[53] 1995 |
United States |
Retrospective |
710 |
4 |
2 |
2 |
— |
— |
50 |
|
Sailhamer[9] 2009 |
United States |
Retrospective |
4,796 |
78 |
69 |
6 |
3 |
X |
35 |
|
Seder[54] 2009 |
United States |
Retrospective |
6,841 |
69 |
68 |
1 |
— |
X |
42 |
|
Synnott[55] 1998 |
Ireland |
Retrospective |
138 |
5 |
5 |
— |
— |
— |
80 |
|
Trudel[56] 1995 |
Canada |
Prospective |
350 |
7 |
7 |
— |
— |
— |
64 |