Damage control resuscitation in patients with severe traumatic hemorrhage
Published 2017
Citation: J Trauma. 82(3):605-617, March 2017
Authors
Cannon, Jeremy W. MD, SM; Khan, Mansoor A. MBBS (Lond), PhD; Raja, Ali S. MD; Cohen, Mitchell J. MD; Como, John J. MD, MPH; Cotton, Bryan A. MD; DuBose, Joseph J. MD; Fox, Erin E. PhD; Inaba, Kenji MD; Rodriguez, Carlos J. DO; Holcomb, John B. MD; Duchesne, Juan C. MD
Author Information
From the Division of Traumatology, Surgical Critical Care & Emergency Surgery (J.W.C.), Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania; Imperial College Healthcare NHS Trust (M.A.K.), London, England; Department of Emergency Medicine (A.S.R.), Massachusetts General Hospital, Boston, MA; Harvard Medical School (A.S.R.), Boston, MA; Department of Surgery (M.J.C.), Denver Health Medical Center, Denver, CO; Department of Surgery (J.J.C.), Metrohealth Medical Center, Cleveland, OH; Division of Acute Care Surgery (B.A.C., E.E.F., J.B.H.), University of Texas Health Science Center at Houston, Houston, TX; Division of Vascular Surgery (J.J.D.), David Grant Medical Center, Travis Air Force Base, CA; Division of Trauma and Critical Care (K.I.), University of Southern California, Los Angeles, CA; General Surgery (C.J.R.), Uniformed Services University-Walter Reed Department of Surgery, Bethesda, MD; North Oaks Shock Trauma Program (J.C.D.), Hammond, LA.
Submitted: August 4, 2016, Revised: October 10, 2016, Accepted: October 12, 2016, Published online: January 3, 2017.
This work was presented in part at the 27th annual meeting of the Eastern Association for the Surgery of Trauma, January 14–18, 2014, in Naples, Florida, and at the 29th annual meeting of the Eastern Association for the Surgery of Trauma, January 12–16, 2016, in San Antonio, Texas.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
Address for reprints: Jeremy W. Cannon, MD, SM, FACS, Penn Presbyterian Medical Center, MOB Suite 120, 51 N 39th St, Philadelphia, PA 19104; email: jeremy.cannon@uphs.upenn.edu.

Table 1. Principles of Damage Control Resuscitation (DCR)
The optimal strategy for managing hemorrhaging trauma patients is now termed damage control resuscitation (DCR) (Table 1).[1–25] Damage control resuscitation seeks to minimize blood loss until definitive hemostasis is achieved.[26–28] Damage control resuscitation has proven successful in combat casualty care,[29][30] prompting the translation of DCR principles to civilian trauma care as well.[31] The following practice management guideline (PMG) quantifies the potential benefits of several aspects of DCR and provides recommendations for managing severely injured patients at risk of death from hemorrhage.
Objectives
The objective of this PMG was to evaluate key components of DCR, including the role of massive transfusion (MT) or DCR protocols, the ratio of plasma (PLAS) and platelets (PLT) to red blood cells (RBC), and the role of hemostatic adjuncts such as recombinant activated factor VII (rVIIa) and tranexamic acid (TXA) in the management of severely injured bleeding patients. Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology,[32] we defined four population (P), intervention (I), comparator (C), and outcome (O) (PICO) questions:
PICO Question 1: In adult patients with severe trauma (P), should an MT/DCR protocol (I) versus no MT/DCR protocol (C) be used to decrease mortality or total blood products used (O)?
PICO Question 2: In adult patients with severe trauma (P), should a high ratio of PLAS and PLT to RBC (I) versus a low ratio (C) be administered to decrease mortality or total blood products (O)?
PICO Question 3: In adult patients with severe trauma (P), should rVIIa (I) versus no rVIIa (C) be administered to decrease mortality, total blood products used, or MT? Does use of rVIIa increase rates of venous thromboembolic events (VTEs) (O)?
PICO Question 4: In adult patients with severe trauma (P), should TXA (I) versus no TXA (C) be administered to decrease mortality, total blood products used, or MT? Does use of TXA increase rates of venous thromboembolic events (VTE) (O)?
Methods
Identification of References
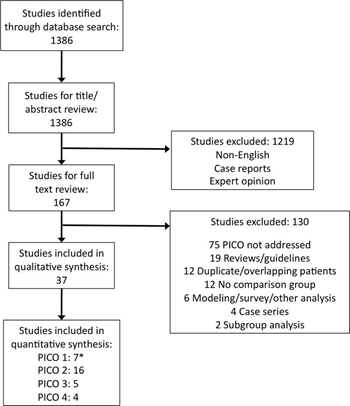
Figure 1. PRISMA diagram of included studies. *Shaz et al.41 considered in PICO 1 and PICO 2.
A systematic review of the medical literature was performed using the , MEDLINE, and EMBASE databases to identify English-language human studies published from January 1985 through December 2015 using the medical subject heading (MeSH) terms and keywords listed (Table, Supplemental Digital Content 1, http://links.lww.com/TA/A860). All studies of adult patients including randomized controlled trials (RCTs), observational studies, and retrospective studies were considered. Severely injured patients at risk of death from hemorrhage were defined as patients requiring blood transfusion and/or injury severity score greater than 25. The literature search was conducted by two authors (M.A.K. and J.C.D.) who then performed title and abstract review to exclude articles in languages other than English, case reports, and expert opinion. Four authors (J.W.C., M.A.K., A.S.R, and J.C.D.) then performed full text review of the remaining articles.
The search generated 1,386 articles. A total of 1,219 were excluded by title and abstract review, leaving 167 articles for full text review. Subsequently, another 130 were excluded, leaving 37 for analysis. Of these, six were used for qualitative analysis only,[19][33–37] while 31 met criteria for quantitative analysis (Fig. 1).[13][14][18][20][23–25][38–61]
Selection of Outcome Measures
Relevant outcomes were identified by four authors (J.W.C., M.A.K., A.S.R., and J.C.D.) including mortality, intensive care unit length of stay, hospital length of stay, total blood products used, need for MT, and specific complications including multisystem organ failure (MSOF), VTE (including deep venous thrombosis and pulmonary embolism), and transfusion reactions. These outcomes were then scored from 1 (less important) to 9 (critically important) (Table, Supplemental Digital Content 2, http://links.lww.com/TA/A861) and those with a score of 7 or greater were considered. For all PICO questions, mortality and total blood products used were deemed critical. For PICO 3 and 4, additional critical outcomes included need for MT and VTE events.
Data Extraction and Methodology
For PICO 1, a total of seven retrospective studies assessed the value of an MT or DCR protocol.[13][14][38–42] One additional study did not define when the MT protocol (MTP) was implemented,[33] while three others described modifications to an existing MT or DCR protocol.[35–37] Consequently, these four studies were considered in the qualitative analysis only.
For PICO 2, the randomized prospective PROPPR study,[20] two prospective, observational studies,[43][44] and 12 retrospective studies[18][41][45–54] evaluated PLAS:RBC ratios. PROPPR[20] and three retrospective studies evaluated PLT:RBC ratios.[41][47][55] PROMMTT[19] used a time-varying analysis that was not conducive to meta-analysis; so it was used for qualitative analysis along with one other study on PLT transfusion.[34]
Hemostatic adjuncts that have been adequately studied for the purposes of this PMG include rVIIa and TXA. While there is increasing interest in the use of prothrombin complex concentrate, fibrinogen concentrate, and desmopressin in managing acutely bleeding trauma patients,[62–64] there is currently insufficient evidence to systematically evaluate their use. For rVIIa, two randomized, prospective, placebo-controlled studies[25][56] and three retrospective studies met inclusion criteria.[57–59] Tranexamic acid was the subject of a large, randomized, prospective, placebo-controlled international trial (CRASH-2) that was included in our analysis.[23] One additional prospective study[60] and the two retrospective Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs and MATTERs II) studies were also included.[24][61] While these military studies contained overlapping patients, only MATTERs reported rates of VTE, a critical outcome. Thus, MATTERs II was used to assess mortality, blood product use, and MT, while MATTERs was used to evaluate VTE.
One author (J.W.C.) entered data from each study into Review Manager (RevMan, Cochrane Collaboration, version 5.2) for quantitative analysis. Forest plots were generated for each critical outcome after calculating the random effects relative risk for categorical variables and mean difference for the one continuous variable (blood product use). Mortality was taken as 28-day, 30-day, or hospital mortality according to each study. Detailed blood product use was infrequently reported, although many studies consistently reported units of RBC given in 24 hours. Thus, this was used as a surrogate end point for total blood products. Because RevMan uses mean difference, when a median value was reported, a normal distribution was assumed.
Evidence profiles were generated for each PICO using GRADEpro GDT (GRADEpro Guideline Development Tool, McMaster University, 2015, available at gradepro.org). We considered study methodology as well as the domains of study bias, inconsistency, indirectness, imprecision, and publication bias when rating the quality of evidence as high, moderate, low, or very low using established methods used by GRADE.[32][65] Implicit consideration was given to the risks and benefits of each intervention along with the most likely values and preferences of patients we have collectively managed in these life-threatening situations. All members of the subcommittee voted on the proposed recommendations for each PICO using Survey Monkey (www.surveymonkey.com) or RedCap electronic data capture tools hosted at the University of Pennsylvania.
Results
Results for MT/DCR Protocol Use (PICO 1)
In adult patients with severe trauma, should an MT/DCR protocol versus no MT/DCR protocol be used to decrease mortality or total blood products used?
Qualitative Synthesis
Transfusion protocols have been used in hospitals for many years.[33][66] Recently, more prescriptive protocols detailing the process of blood bank notification and the mix of blood products delivered to the bedside have emerged.[18][67–69] These MTPs, which may also emphasize hypothermia prevention, minimizing crystalloid, and permitting modest hypotension, have now become integral to the overall paradigm of DCR.[70–72]
A total of 11 retrospective studies have evaluated the implementation of an MTP with or without a formal DCR protocol.[13][14][33][35–42] On qualitative analysis, there was significant variability in the MTPs described in terms of the numbers of products provided and the preplanned product ratios, which ranged from PLAS:PLT:RBC as low as 2:0:5[13] to as high as 2:1:2.[39][41] Only one study detailed specific MTP activation criteria beyond attending surgeon judgment.[39]
All of the included studies noted benefits to the use of an MTP measured by improved patient survival,[14][35][39][40] decreased use of blood products,[35][40] or cost savings.[13] One paper further reported reduced MSOF and postresuscitation complications including abdominal compartment syndrome.[35] The one military paper in this group demonstrated increased use of blood products, particularly PLAS, PLT, and cryoprecipitate; however, MTP use resulted in earlier physiologic recovery.[42] Finally, MTP use seemed to minimize or even reduce blood product wastage.[36][39]
Quantitative Synthesis (Meta-analysis)
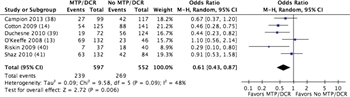
Figure 2. Forest plot for MT/DCR protocol vs no MT/DCR protocol; outcome = mortality.
Seven studies met criteria for quantitative analysis.[13][14][38–42] In six retrospective studies with a total of 1,149 patients, the mean mortality for patients managed with an MT/DCR protocol was 40.0% compared to 48.7% with no protocol. The relative risk for either 30-day or hospital mortality was 0.61 (confidence interval [CI], 0.43–0.87; p = 0.006) (Fig. 2; Table, Supplemental Digital Content 3, http://links.lww.com/TA/A862).
Four studies reported total RBC units transfused in 24 hours in a total of 511 patients.[13][40–42] This was used as a surrogate for total blood product use as previously described. Quantitatively, patients managed with an MT/DCR protocol received similar numbers of RBC units as those managed without an MTP (Figure, Supplemental Digital Content 4, http://links.lww.com/TA/A863; Table, Supplemental Digital Content 3, http://links.lww.com/TA/A862).
Grading the Evidence
The overall quality of this evidence was found to be low (mortality) to very low (RBC use) owing to the retrospective design of the studies considered with a serious risk of bias and the inconsistency, indirectness, and imprecision in the blood product administration analyses (Table, Supplemental Digital Content 3, http://links.lww.com/TA/A862). No evidence of publication bias was identified (Figure, Supplemental Digital Content 5, http://links.lww.com/TA/A864).
Recommendation
In formulating a recommendation for the implementation of PICO 1, we considered that many patients with hemorrhage presenting to a trauma center would place a high value on a rapid and well-coordinated resuscitation effort focused on arresting hemorrhage, reversing shock, and preventing coagulopathy. The risks of applying an MT/DCR protocol seem to be low, and use of an MT/DCR protocol is associated with a significant survival benefit. Based on this evidence, eight authors (73%) voted for a strong recommendation, while three (27%) voted for a conditional recommendation. Thus, we recommend the development and implementation of an MT/DCR protocol for the management of severely injured trauma patients. This should be done with multidisciplinary input using the most current evidence to guide indications for protocol activation,[73] the target blood product ratios implicit within the protocol, and the many other aspects of MT/DCR protocol implementation.[74]
Results for PLAS:RBC and PLT:RBC (PICO 2)
In adult patients with severe trauma, should a high ratio of PLAS:RBC and PLT:RBC versus a low ratio be administered to decrease mortality or total blood products used?
Qualitative Synthesis
Before the 1970s, whole blood was the resuscitation fluid of choice for bleeding trauma patients.[75] However, routine separation of whole blood into components for storage and renewed interest in crystalloid-based resuscitation shifted practice toward infusing large volumes of crystalloid and many units of RBC before considering PLAS or PLT transfusion.[76] Unfortunately, this approach caused dilutional coagulopathy and many other complications in the most severely injured patients.
The landmark publication by Borgman et al.[18] examining the role of a more balanced transfusion strategy in combat casualties demonstrated improved survival. Numerous subsequent reports have suggested that early transfusion of PLAS and RBC in a balanced ratio of 1:1 to 1:1.5 is associated with lower mortality and less MSOF in patients who receive an MT.[47][77] Some have suggested the mortality may increase in a U-shaped curve as the ratio approaches 1:1,[50][78][79] while others have preliminarily found that ratios above 1:1 (i.e., more PLAS than RBC) may be associated with improved short-term survival.[54] Empiric PLT transfusion has also been evaluated to determine the most effective ratio of PLT:RBC. Both single-center[34] and multicenter studies[80] have demonstrated a stepwise improvement in survival among MT patients with increasing PLT:RBC ratios.
We systematically evaluated a total of 18 studies on this topic qualitatively. Of these, 16 met criteria for quantitative synthesis, including one RCT, which was recently completed,[20] two prospective observational studies,[43][44] and 13 retrospective studies.[18][41][45–55] Two additional studies were considered in the qualitative analysis.[19][34]
We defined a high ratio of PLAS:RBC and PLT:RBC as close as possible to 1:1:1 (relatively more PLAS and PLT). Conversely, we defined a low ratio as less than or equal to 1:1:2 (relatively less PLAS and PLT). Some studies did not fit precisely into this categorization scheme. Specifically, three studies had groups that crossed the 1:2 boundary.[43][44][46] To remain consistent in our analysis, we included these studies in the analysis but categorized all boundary patients as receiving a low ratio to weight any potentially favorable findings toward a low-ratio strategy (Figure, Supplemental Digital Content 6, http://links.lww.com/TA/A865).
In theory, the effect of a particular ratio of PLAS:RBC can be distinguished from the effect of the PLT or RBC ratio and other hemostatic agents given during resuscitation. However, very few studies on blood product ratios have specifically accounted for these confounding factors, since all three products may be infusing simultaneously during an active resuscitation.[47] A recent review of transfusion practices in combat casualty care actually identified a synergistic effect between a high ratio of PLAS:RBC and a high ratio of PLT:RBC.[81] Thus, to fully distinguish the effect of PLAS from PLT ratios, a 2 × 2 RCT factorial study design would be required.
Another point where our thinking about blood product ratios has evolved is the timing of ratio calculations. To date, most studies have calculated the ratio of blood products used for a particular resuscitation at 24 hours.[18][41][49] However, in reality, transfusion ratios fluctuate throughout a resuscitation, a dynamic element that retrospective studies are unable to capture.[19][82] To better inform clinical practice, future studies on blood product ratios should assess ratios much sooner than 24 hours, likely within 2 to 4 hours[19][50][52]and, most importantly, at the time of surgical hemostasis.
The qualitative analysis indicated an early mortality benefit to targeting a high ratio of PLAS and PLT:RBC[19] due to a more frequent achievement of hemostasis[20] and decreased death from truncal hemorrhage[47] or exsanguination.[20] Indeed, death from hemorrhage was significantly decreased at 24 hours in the 1:1:1 group compared to even 1:1:2 in the PROPPR study.[20] Assessment of the impact of a high ratio of PLAS and PLT on total blood products transfused, RBC transfused, and patients receiving MT was limited by the few studies reporting these end points. Multisystem organ failure was reported in four studies with no significant increase with a high ratio of PLAS and/or PLT. One RCT also found that transfusion reactions as well as 23 prespecified complications (including acute respiratory distress syndrome, acute kidney injury, and MSOF) were not greater in the high-ratio group despite more PLAS and PLT given.[20] One transfusion-related death (due to transfusion-associated circulatory overload) was reported in the 1:1:1 group at 30 days.
Quantitative Synthesis (Meta-analysis)
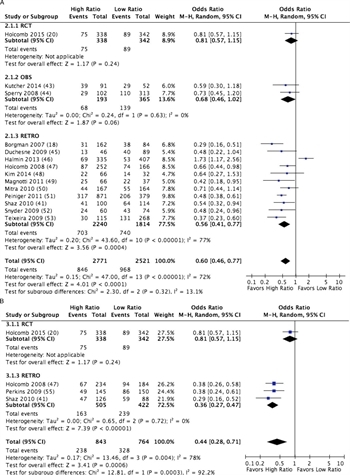
Figure 3. forest plot for high vs low ratio PLAS:RBC (A) and PLT:RBC (B); outcome = mortality.
A total of 15 studies met criteria for quantitative analysis of high versus low PLAS:RBC ratios,[18][20][41][43–54] and four met criteria for analysis of PLT:RBC ratios.[20][41][47][55] A total of 2,771 patients received a high ratio of PLAS:RBC, and 2,521 received a low ratio of PLAS:RBC, for a total of 5,292 patients studied. Mortality was 30.5% in the high-ratio group as compared to 38.4% in the low-ratio group with an odds ratio of 0.60 (CI, 0.46–0.77; p < 0.0001) (Fig. 3A; Table, Supplemental Digital Content 7, http://links.lww.com/TA/A866). When considering only the RCT and the observational data, an all-cause mortality difference was still observed at 30 days or hospital discharge. Furthermore, germane to the purpose of DCR, these studies all demonstrated significantly fewer deaths from hemorrhage using a high-ratio strategy.
For PLT:RBC ratios, 843 patients received a high ratio and 764 received a low ratio, for a total of 1,607 patients studied. Mortality was 28.2% in the high-ratio group compared to 42.9% in the low-ratio group, with an odds ratio of 0.44 (CI, 0.28–0.71; p = 0.0003) (Fig. 3B; Table, Supplemental Digital Content 7, http://links.lww.com/TA/A866).
Five studies met criteria for evaluating blood product use in high versus low PLAS:RBC,[20][43][44][48][54] while only one met criteria for this same end point in high versus low PLT:RBC.[20] No significant difference in RBC given was identified between the high- and low-ratio groups (Figure, Supplemental Digital Content 8, http://links.lww.com/TA/A867; Table, Supplemental Digital Content 7, http://links.lww.com/TA/A866).
Grading the Evidence
At baseline, the quality of the evidence evaluated for this PICO question was considered moderate owing to the presence of an RCT (high quality) and two observational studies (moderate quality), which balanced multiple retrospective studies with low to very low quality of evidence due to a serious risk of bias. Heterogeneity between these study groups was moderate for PLAS:RBC data and high for PLT:RBC data. Publication bias was considered unlikely for the PLAS:RBC data (Figure, Supplemental Digital Content 9, http://links.lww.com/TA/A868).
Survival bias has also been raised as a concern by those evaluating the potential risks and benefits of a high-ratio resuscitation strategy.[19][52][83] This bias results when early death precludes administration of a full complement of blood products. If the initial products given were RBC, the patient would be classified as a low-ratio patient although the patient may have died regardless of the transfusion strategy used. The only way to effectively eliminate this bias is to evaluate blood product ratios as time-varying covariates[19][52] or in a randomized controlled manner[20] with equal and simultaneous availability of all three components. The inclusion of multiple studies that accounted for this survival bias and the fact that we included patients on the boundary of the high and low strategies in the low group minimized the possibility of such bias in the overall analysis.
The mortality benefit we found for a high ratio of PLAS:RBC was very large, offsetting downgrades for inclusion of retrospective and observational studies and for inconsistency, thus justifying an upgrade in the overall quality of evidence to moderate (Table, Supplemental Digital Content 7, http://links.lww.com/TA/A866). For PLT:RBC, the mortality benefit was also very large, which offset downgrades for the risk of bias due to inclusion of retrospective and observational studies and partially offset the high heterogeneity in the results between studies signifying serious inconsistency. Thus, we graded the overall quality of evidence for these data as low (Table, Supplemental Digital Content 7, http://links.lww.com/TA/A866).
Recommendation
We believe patients in hemorrhagic shock would value rapid reversal of this condition using the most effective resuscitation strategy available. Transfusion therapy for acute hemorrhage has become much safer than ever before with minimal risk of infectious disease transmission or adverse reaction. From a layperson's standpoint, treatment of hemorrhagic shock should involve the replacement of shed blood with products that functionally resemble what has been lost. Thus, we believe most patients would value a high-ratio DCR strategy, if not whole blood (which remains Food and Drug Administration and AABB approved).[84–86]
Based on the available evidence indicating a significant benefit to a high ratio of PLAS:RBC, nine members (82%) voted for a strong recommendation and two (18%) voted for a weak recommendation. Regarding a high ratio of PLT:RBC, eight (73%) voted for a strong recommendation, while three (27%) voted for a conditional recommendation. Thus, we recommend targeting a high ratio of both PLAS and PLT:RBC for resuscitating severely injured bleeding trauma patients. Preparing MT packs or pre-positioning blood products in the trauma resuscitation bay in a 1:1:1 ratio (e.g., 6 units PLAS, 1 unit apheresis PLT, and 6 units RBC) can help avoid a significant ratio imbalance during the early empiric resuscitation phase. Additionally, leading with hemostatic PLAS and PLT early and then catching up with RBC in short order seems to be a safe guiding principle,[82]although further data are needed in this area.
Results for rVIIa (PICO 3)
In adult patients with severe trauma, should the hemostatic adjunct rVIIa versus no rVIIa be administered to decrease mortality, total blood products used, or MT? Does use of rVIIa increase rates of VTE?
Qualitative Synthesis
Recombinant activated factor VII has been used as a pharmacologic adjunct to promote hemostasis in the setting of uncontrolled hemorrhage since 1999,[87] although this remains an off-label use in the United States. Two RCTs[25][56] and three retrospective studies (including one case-control study)[57–59] evaluated the use of rVIIa specifically for trauma and met criteria for qualitative analysis. The dose of rVIIa studied in the RCTs was 200 μg/kg initially followed by 100 μg/kg at Hours 1 and 3. Other doses have also been evaluated including “low-dose” ranging from 40 μg/kg down to 1.2 mg (approximately 15 μg/kg) for management of mild to moderate coagulopathy following injury.[57][88]
Critical outcomes included hospital mortality (in-hospital, 28-day, or 30-day), blood products administered, the need for MT, and development of VTE. Qualitative analysis indicated no mortality benefit for rVIIa, and blood product use was no different with or without use of rVIIa. However, use of rVIIa may reduce the need for MT in some patients.[56]
Patient follow-up in the studies on rVIIa ranged from hospital discharge (or arrival at a Level V combat facility)[56–59] to 90 days after discharge.[25] Venous thromboembolic event surveillance timing, frequency, and methods were not standardized or reported in any of these studies. With these limitations in mind, there was no evidence of increased rates of VTE on qualitative assessment.
Quantitative Synthesis (Meta-analysis)
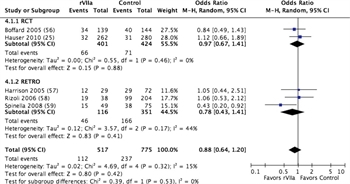
Figure 4. Forest plots for rVIIa vs no rVIIa; outcome = mortality.
These same five studies were also considered for quantitative analysis of rVIIa. A total of 517 patients received rVIIa compared to 775 who did not, for a total of 1,292 patients studied. Mortality was 21.7% in patients receiving rVIIa and 30.6% in those who did not receive rVIIa, although this difference was not statistically significant with a relative risk of 0.88 (CI, 0.64–1.2; p = 0.42) (Figure 4; Table, Supplemental Digital Content 10, http://links.lww.com/TA/A869). There was no difference in units of RBC transfused, although fewer MTs were needed in patients receiving rVIIa (Figure, Supplemental Digital Content 11, http://links.lww.com/TA/A870; Table, Supplemental Digital Content 10, http://links.lww.com/TA/A869). Within the limitations previously noted, VTE rates were no greater with rVIIa administration compared to controls (Figure, Supplemental Digital Content 9, http://links.lww.com/TA/A868).
Grading the Evidence
The baseline quality of evidence based on study methodology was considered moderate for rVIIa. For mortality, inclusion of retrospective studies with moderate to high risk of bias downgraded the quality. Otherwise, the results were homogeneous across all end points except for VTE, resulting in a final quality assessment of low to moderate for rVIIa (Table, Supplemental Digital Content 10, http://links.lww.com/TA/A869).
Recommendation
For most bleeding trauma patients, there does not seem to be a clear, significant mortality benefit from rVIIa. If given early in the resuscitation, rVIIa may decrease the need for a MT. Although there is also no evidence that rVIIa leads to more VTEs, this end point has not been well evaluated in the trauma population. One study in a mixed population of critically ill patients did demonstrate an increased rate of arterial thrombosis with rVIIa administration.[89] Thus, we believe most patients would want these agents given on a selective basis, reserved for those with significant hemorrhage and severe injuries.
The subcommittee was divided on the best recommendation based on this evidence. Four (36%) voted for a weak recommendation for rVIIa, while two (18%) voted against rVIIa (one weak and one strong) and five (45%) felt the data did not support any recommendation for or against rVIIa. Thus, we cannot recommend for or against the use of rVIIa in the management of severely injured adult trauma patients. This adjunct does not seem to improve all-cause mortality across all patient populations, and its only demonstrated benefit is a possible reduced need for MT. We feel that the use of rVIIa needs further study with particular attention to optimal dosing and the timing of rVIIa relative to blood product administration before a recommendation for or against its use can be made. Furthermore, VTE rates need to be more carefully evaluated with a defined surveillance protocol in future studies.
Results for TXA (PICO 4)
In adult patients with severe trauma, should the hemostatic adjunct TXA versus no TXA be administered to decrease mortality, total blood products used, or MT? Does use of TXA increase rates of VTE?
Qualitative Synthesis
Fibrinogen is a critical precursor for clot formation. In trauma patients, fibrinogen is lost in shed blood and can be rapidly consumed through the activation of in vivo hemostatic mechanisms. Excessive and inappropriate fibrinolysis can also lead to further fibrinogen consumption as part of the coagulopathy of trauma.[90] This process can be blocked with antifibrinolytic agents such as TXA or aminocaproic acid (Amicar), although a mechanistic link between antifibrinolysis and improved outcomes has not been established.[91][92]Use of these medications in trauma patients is currently considered off-label.
A total of four studies addressed TXA use in a hospital setting.[23][24][60][61] The analysis of TXA was dominated by the international CRASH-2 RCT.[23] The other studies on TXA use in trauma that met criteria for analysis included a recently published prospective study in civilians[60] and the retrospective combat casualty experience with this agent in Afghanistan reported in MATTERs and MATTERs II.[24][61] The CRASH-2 and MATTERs studies include patients with significant disparities in mechanism and injury severity. In CRASH-2, 68% of patients had a blunt mechanism of injury compared to MATTERs, which reported only patients injured by gunshot wound (30%) or explosion (70%). In CRASH-2, injury severity was not reported; however, only 18% had a Glasgow Coma Scale score of 8 or less compared to 29% in MATTERs. Additionally, less than half of CRASH-2 patients had a transfusion or required surgery. These findings lead us to question the applicability of CRASH-2 to severely injured, bleeding trauma patients.
Critical outcomes evaluated in patients managed with TXA included hospital mortality (in-hospital, 28-day, or 30-day), blood products administered, the need for MT, and development of VTE. Qualitative analysis indicated only a modest benefit for TXA in the most severely injured patients with clear evidence of bleeding. Tranexamic acid should be given early, as mortality significantly increases if given more than 3 hours after injury.[93] Blood product use was no different with or without use of TXA. Again, VTE surveillance timing, frequency, and methods were not standardized or reported in any of the studies considered.
Quantitative Synthesis (Meta-analysis)
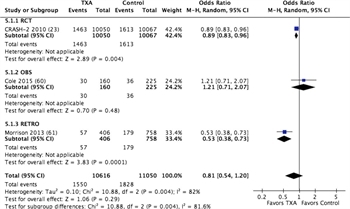
Figure 5. Forest plots for TXA vs no TXA; outcome = mortality.
These same four studies met criteria for quantitative analysis. There was no demonstrable difference in mortality between patients who received TXA (14.6%) and those who did not (16.5%), with a relative risk of 0.7 (CI, 0.54–1.2; p = 0.29) (Fig. 5; Table, Supplemental Digital Content 12, http://links.lww.com/TA/A871). There was also no difference in units of RBC transfused in those who received TXA versus those who received no TXA (Figure, Supplemental Digital Content 13, http://links.lww.com/TA/A872; Table, Supplemental Digital Content 12, http://links.lww.com/TA/A871). The one study that reported MT use in TXA was a combat study in which TXA was part of the MT protocol (Figure, Supplemental Digital Content 13, http://links.lww.com/TA/A872). Venous thromboembolic events were likely underdiagnosed in the RCT considered with no difference between groups identified,[23] while they were more frequent in the TXA group in the MATTERs study.[24] However, in aggregate, within the limits of this end point previously noted, VTEs were no greater with TXA administration (Figure, Supplemental Digital Content 13, http://links.lww.com/TA/A872).
Grading the Evidence
The quality of evidence for TXA was considered moderate at baseline; however, the inclusion of retrospective data, limitations of the CRASH-2 data, and findings of inconsistency and imprecision downgraded the evidence to very low (Table, Supplemental Digital Content 12, http://links.lww.com/TA/A871).
Recommendation
The evidence for in-hospital use of TXA demonstrates a mortality benefit in a mixed population of questionably bleeding trauma patients in one international RCT,[23] on subgroup analysis of a prospectively studied group of severely injured civilian patients in shock,[60] and on retrospective review of severely injured combat casualties.[24][61] When these results are combined, there is no clear universal mortality benefit to TXA; however, the safety profile of this medication seems to be favorable when used early after injury (i.e., within 3 hours). Seven subcommittee members (64%) supported a conditional recommendation for TXA use, while one (9%) favored a strong recommendation and three (27%) felt that a recommendation could not be made for or against TXA use. Thus, we conditionally recommend TXA use as a hemostatic adjunct in the management of severely injured adult trauma patients. These recommendations apply only to the use of TXA in a hospital setting pending the results of two ongoing prehospital TXA trials. As with other hemostatic agents, VTE rates need to be more carefully evaluated with the use of a defined surveillance protocol in future studies on TXA.
Discussion
Based on this focused analysis, there is compelling evidence that a well-planned MT/DCR protocol will improve survival without increasing blood product usage. Indeed, implementing an MTP is now required of all trauma centers verified by the American College of Surgeons.[94] Furthermore, a high ratio of PLAS and PLT to RBC reduces hemorrhage-related mortality and likely also reduces all-cause mortality, a finding consistent with previous systematic reviews[77] and published guidelines.[63][95][96] We believe this is best achieved by transfusing equal parts of RBC, PLAS, and PLT early during resuscitation, transitioning to a laboratory-based resuscitation strategy as results become available.[20] Finally, these benefits may be further augmented by the early in-hospital use of TXA in severely injured bleeding patients.
Using this Guideline in Clinical Practice
The recommendations in this PMG are the result of a comprehensive and systematic analysis of the literature on several aspects of the DCR paradigm. Although the GRADE approach attempts to overcome some limitations of a meta-analysis with a transparent qualitative assessment and evidence evaluation process, these recommendations should not replace clinical judgment.
Conclusion

TABLE 2 Summary of Recommendations Confirm
In this PMG, we offer four evidence-based recommendations on DCR in the management of severely injured bleeding trauma patients (Table 2). We recommend the development and routine use of an MT/DCR protocol for severely injured patients that intrinsically targets a high ratio of PLAS and PLT:RBC. We cannot recommend for or against the use of rVIIa at this time, but we conditionally recommend TXA early in the management of these patients.
Authorship
J.W.C. and A.S.R. served as EAST Guideline Section liaisons. J.J.C. served as the EAST Trauma Guideline Taskforce leader. J.W.C., M.A.K., A.S.R., and J.C.D. formulated the PICO questions. M.A.K. and J.C.D. conducted the literature search. M.A.K. screened titles and abstracts. J.W.C., M.A.K., A.S.R., and J.C.D. performed the full text review. J.W.C. abstracted data from selected articles. J.W.C., M.A.K., A.S.R., and J.C.D. coordinated the systematic review. All authors appraised the evidence and contributed to the final recommendations. J.W.C., M.A.K., and J.C.D. drafted the initial manuscript, which all authors critically revised.
Acknowledgement
The authors gratefully acknowledge the collaboration of Bryce H. Robinson, MD, from the University of Washington, Seattle, WA and Elliott R. Haut, MD, PhD, from The Johns Hopkins School of Medicine, Baltimore, MD.
The authors also thank GRADE expert Rebecca Morgan for she insights she provided in performing the analysis for this guideline.
Disclosure
The author declares no conflicts of interest.
References
- Gentilello LM, Jurkovich GJ, Stark MS, Hassantash SA, O'Keefe GE. Is hypothermia in the victim of major trauma protective or harmful? A randomized, prospective study. Ann Surg. 1997;226(4):439–447.
- Shafi S, Elliott AC, Gentilello L. Is hypothermia simply a marker of shock and injury severity or an independent risk factor for mortality in trauma patients? Analysis of a large national trauma registry. J Trauma. 2005;59(5):1081–1085.
- Kragh JF Jr, Walters TJ, Baer DG, Fox CJ, Wade CE, Salinas J, Holcomb JB. Survival with emergency tourniquet use to stop bleeding in major limb trauma. Ann Surg. 2009;249(1):1–7.
- Schroll R, Smith A, McSwain NE Jr, Myers J, Rocchi K, Inaba K, Siboni S, Vercruysse GA, Ibrahim-zada I, Sperry JL, et al. A multi-institutional analysis of prehospital tourniquet use. J Trauma Acute Care Surg. 2015;79(1):10–14.
- Inaba K, Siboni S, Resnick S, Zhu J, Wong MD, Haltmeier T, Benjamin E, Demetriades D. Tourniquet use for civilian extremity trauma. J Trauma Acute Care Surg. 2015;79(2):232–237; quiz 332–3.
- Leonard J, Zietlow J, Morris D, Berns K, Eyer S, Martinson K, Jenkins D, Zietlow S. A multi-institutional study of hemostatic gauze and tourniquets in rural civilian trauma. J Trauma Acute Care Surg. 2016;81(3):441–444.
- Yong E, Vasireddy A, Pavitt A, Davies GE, Lockey DJ. Pre-hospital pelvic girdle injury: improving diagnostic accuracy in a physician-led trauma service. Injury. 2016;47(2):383–388.
- DuBose JJ, Scalea TM, Brenner M, Skiada D, Inaba K, Cannon J, Moore L, Holcomb J, Turay D, Arbabi CN, et al. The AAST prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry: data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016;81(3):409–419.
- Bickell WH, Wall MJ, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331(17):1105–1109.
- Dutton RP, Mackenzie CF, Scalea TM. Hypotensive resuscitation during active hemorrhage: impact on in-hospital mortality. J Trauma Acute Care Surg. 2002;52(6):1141–1146.
- Duchesne JC, Heaney J, Guidry C, McSwain N Jr, Meade P, Cohen M, Schreiber M, Inaba K, Skiada D, Demetriades D, et al. Diluting the benefits of hemostatic resuscitation: a multi-institutional analysis. J Trauma Acute Care Surg. 2013;75(1):76–82.
- Schreiber MA, Meier EN, Tisherman SA, Kerby JD, Newgard CD, Brasel K, Egan D, Witham W, Williams C, Daya M, et al. A controlled resuscitation strategy is feasible and safe in hypotensive trauma patients: results of a prospective randomized pilot trial. J Trauma Acute Care Surg. 2015;78(4):687–695.
- O'Keeffe T, Refaai M, Tchorz K, Forestner JE, Sarode R. A massive transfusion protocol to decrease blood component use and costs. Arch Surg. 2008;143(7):686–690.
- Cotton BA, Au BK, Nunez TC, Gunter OL, Robertson AM, Young PP. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma. 2009;66(1):41–48.
- Meizoso JP, Ray JJ, Karcutskie CA, Allen CJ, Zakrison TL, Pust GD, Koru-Sengul T, Ginzburg E, Pizano LR, Schulman CI, et al. Effect of time to operation on mortality for hypotensive patients with gunshot wounds to the torso: the golden 10 minutes. J Trauma Acute Care Surg. 2016;81(4):685–691.
- Schwartz DA, Medina M, Cotton BA, Rahbar E, Wade CE, Cohen AM, Beeler AM, Burgess AR, Holcomb JB. Are we delivering two standards of care for pelvic trauma? Availability of angioembolization after hours and on weekends increases time to therapeutic intervention. J Trauma Acute Care Surg. 2014;76(1):134–139.
- Tesoriero RB, Bruns BR, Narayan M, Dubose J, Guliani SS, Brenner ML, Boswell S, Stein DM, Scalea TM. Angiographic embolization for hemorrhage following pelvic fracture: is it “time” for a paradigm shift? J Trauma Acute Care Surg. 2017;82(1):18–26.
- Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–813.
- Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127.
- Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482.
- Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051–1059.
- Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall MJ Jr, Mattox KL, Suliburk J. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74(2):378–385; discussion 385–6.
- CRASH-2 trial collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32.
- Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military application of tranexamic acid in trauma emergency resuscitation (MATTERs) study. Arch Surg. 2012;147(2):113–119.
- Hauser CJ, Boffard K, Dutton R, Bernard GR, Croce MA, Holcomb JB, Leppaniemi A, Parr M, Vincent J-L, Tortella BJ, et al. Results of the CONTROL trial: efficacy and safety of recombinant activated factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69(3):489–500.
- Hodgetts TJ, Mahoney PF, Kirkman E. Damage control resuscitation. J R Army Med Corps. 2007;153(4):299–300.
- Hodgetts TJ, Mahoney PF, Russell MQ, Byers M. ABC to < C > ABC: redefining the military trauma paradigm. Emerg Med J. 2006;23(10):745–746.
- Moore EE, Burch JM, Franciose RJ, Offner PJ, Biffl WL. Staged physiologic restoration and damage control surgery. World J Surg. 1998;22(12):1184–1190; discussion 1190–1.
- Holcomb JB. The 2004 Fitts lecture: current perspective on combat casualty care. J Trauma. 2005;59(4):990–1002.
- Rasmussen TE, Gross KR, Baer DG. Where do we go from here? Preface. US Military Health System Research Symposium, August 2013. J Trauma Acute Care Surg. 2013;75(2 Suppl 2):S105–S106.
- Duchesne JC, McSwain NE, Cotton BA, Hunt JP, Dellavolpe J, Lafaro K, Marr AB, Gonzalez EA, Phelan HA, Bilski T, et al. Damage control resuscitation: the new face of damage control. J Trauma. 2010;69(4):976–990.
- Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, Dahm P, Eastern Association for the Surgery of Trauma Practice Management Guidelines Ad Hoc Committee. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S283–S287.
- Cinat ME, Wallace WC, Nastanski F, West J, Sloan S, Ocariz J, Wilson SE. Improved survival following massive transfusion in patients who have undergone trauma. Arch Surg. 1999;134(9):964–968; discussion 968–70.
- Inaba K, Lustenberger T, Rhee P, Holcomb JB, Blackbourne LH, Shulman I, Nelson J, Talving P, Demetriades D. The impact of platelet transfusion in massively transfused trauma patients. J Am Coll Surg. 2010;211(5):573–579.
- Cotton BA, Reddy N, Hatch QM, LeFebvre E, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Holcomb JB. Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg. 2011;254(4):598–605.
- Khan S, Allard S, Weaver A, Barber C, Davenport R, Brohi K. A major haemorrhage protocol improves the delivery of blood component therapy and reduces waste in trauma massive transfusion. Injury. 2013;44(5):587–592.
- Nascimento B, Callum J, Tien H, Rubenfeld G, Pinto R, Lin Y, Rizoli S. Effect of a fixed-ratio (1:1:1) transfusion protocol versus laboratory-results-guided transfusion in patients with severe trauma: a randomized feasibility trial. CMAJ. 2013;185(12):E583–E589.
- Campion EM, Pritts TA, Dorlac WC, Nguyen AQ, Fraley SM, Hanseman D, Robinson BR. Implementation of a military-derived damage-control resuscitation strategy in a civilian trauma center decreases acute hypoxia in massively transfused patients. J Trauma Acute Care Surg. 2013;75(2 Suppl 2):S221–S227.
- Duchesne JC, Kimonis K, Marr AB, Rennie KV, Wahl G, Wells JE, Islam TM, Meade P, Stuke L, Barbeau JM, et al. Damage control resuscitation in combination with damage control laparotomy: a survival advantage. J Trauma. 2010;69(1):46–52.
- Riskin DJ, Tsai TC, Riskin L, Hernandez-Boussard T, Purtill M, Maggio PM, Spain DA, Brundage SI. Massive transfusion protocols: the role of aggressive resuscitation versus product ratio in mortality reduction. J Am Coll Surg. 2009;209(2):198–205.
- Shaz BH, Dente CJ, Nicholas J, MacLeod JB, Young AN, Easley K, Ling Q, Harris RS, Hillyer CD. Increased number of coagulation products in relationship to red blood cell products transfused improves mortality in trauma patients. Transfusion. 2010;50(2):493–500.
- Fox CJ, Gillespie DL, Cox ED, Mehta SG, Kragh JF Jr, Salinas J, Holcomb JB. The effectiveness of a damage control resuscitation strategy for vascular injury in a combat support hospital: results of a case control study. J Trauma. 2008;64(Suppl 2):S99–S106.
- Kutcher ME, Kornblith LZ, Vilardi RF, Redick BJ, Nelson MF, Cohen MJ. The natural history and effect of resuscitation ratio on coagulation after trauma: a prospective cohort study. Ann Surg. 2014;260(6):1103–1111.
- Sperry JL, Ochoa JB, Gunn SR, Alarcon LH, Minei JP, Cuschieri J, Rosengart MR, Maier RV, Billiar TR, Peitzman AB, et al. An FFP: PRBC transfusion ratio ≥1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65(5):986–993.
- Duchesne JC, Islam TM, Stuke L, Timmer JR, Barbeau JM, Marr AB, Hunt JP, Dellavolpe JD, Wahl G, Greiffenstein P, et al. Hemostatic resuscitation during surgery improves survival in patients with traumatic-induced coagulopathy. J Trauma. 2009;67(1):33–37.
- Halmin M, Boström F, Brattström O, Lundahl J, Wikman A, Östlund A, Edgren G. Effect of plasma-to-rbc ratios in trauma patients: a cohort study with time-dependent data. Crit Care Med. 2013;41(8):1905–1914.
- Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–458.
- Kim Y, Lee K, Kim J, Kim J, Heo Y, Wang H, Lee K, Jung K. Application of damage control resuscitation strategies to patients with severe traumatic hemorrhage: review of plasma to packed red blood cell ratios at a single institution. J Korean Med Sci. 2014;29(7):1007–1011.
- Magnotti LJ, Zarzaur BL, Fischer PE, Williams RF, Myers AL, Bradburn EH, Fabian TC, Croce MA. Improved survival after hemostatic resuscitation: does the emperor have no clothes? J Trauma. 2011;70(1):97–102.
- Mitra B, Mori A, Cameron PA, Fitzgerald M, Paul E, Street A. Fresh frozen plasma (FFP) use during massive blood transfusion in trauma resuscitation. Injury. 2010;41(1):35–39.
- Peiniger S, Nienaber U, Lefering R, Braun M, Wafaisade A, Wutzler S, Borgmann M, Spinella PC, Maegele M. Balanced massive transfusion ratios in multiple injury patients with traumatic brain injury. Crit Care. 2011;15(1):1.
- Snyder CW, Weinberg JA, McGwin G Jr, Melton SM, George RL, Reiff DA, Cross JM, Hubbard-Brown J, Rue LW 3rd, Kerby JD. The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma. 2009;66(2):358–364.
- Teixeira PG, Inaba K, Shulman I, Salim A, Demetriades D, Brown C, Browder T, Green D, Rhee P. Impact of plasma transfusion in massively transfused trauma patients. J Trauma. 2009;66(3):693–697.
- Guidry C, DellaVope J, Simms E, Heaney JB, Guice J, McSwain N Jr, Meade P, Duchesne JC. Impact of inverse ratios on patients with exsanguinating vascular injuries: should more be the new paradigm? J Trauma Acute Care Surg. 2013;74(2):403–410.
- Perkins JG, Cap AP, Spinella PC, Blackbourne LH, Grathwohl KW, Repine TB, Ketchum L, Waterman P, Lee RE, Beekley AC, et al. An evaluation of the impact of apheresis platelets used in the setting of massively transfused trauma patients. J Trauma. 2009;66(Suppl 4):S77–S84.
- Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, Axelsen M, Kluger Y. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma. 2005;59(1):8–15.
- Harrison TD, Laskosky J, Jazaeri O, Pasquale MD, Cipolle M. “Low-dose” recombinant activated factor vii results in less blood and blood product use in traumatic hemorrhage. J Trauma. 2005;59(1):150–154.
- Rizoli SB, Nascimento B, Osman F, Netto FS, Kiss A, Callum J, Brenneman FD, Tremblay L, Tien HC. Recombinant activated coagulation factor VII and bleeding trauma patients. J Trauma. 2006;61(6):1419–1425.
- Spinella PC, Perkins JG, McLaughlin DF, Niles SE, Grathwohl KW, Beekley AC, Salinas J, Mehta S, Wade CE, Holcomb JB. The effect of recombinant activated factor VII on mortality in combat-related casualties with severe trauma and massive transfusion. J Trauma. 2008;64(2):286–294.
- Cole E, Davenport R, Willett K, Brohi K. Tranexamic acid use in severely injured civilian patients and the effects on outcomes: a prospective cohort study. Ann Surg. 2015;261(2):390–394.
- Morrison JJ, Ross JD, Dubose JJ, Jansen JO, Midwinter MJ, Rasmussen TE. Association of cryoprecipitate and tranexamic acid with improved survival following wartime injury: findings from the MATTERs II study. JAMA Surg. 2013;148(3):218–225.
- Schöchl H, Nienaber U, Hofer G, Voelckel W, Jambor C, Scharbert G, Kozek-Langenecker S, Solomon C. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14(2):R55.
- Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E, Filipescu D, Hunt BJ, Komadina R, Nardi G, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20:100.
- Wafaisade A, Lefering R, Maegele M, Brockamp T, Mutschler M, Lendemans S, Banerjee M, Bouillon B, Probst C Trauma Registry of DGU. Administration of fibrinogen concentrate in exsanguinating trauma patients is associated with improved survival at 6 hours but not at discharge. J Trauma Acute Care Surg. 2013;74(2):387–383; discussion 393–5.
- Singh S, Haut ER, Brotman DJ, Sharma R, Chelladurai Y, Shermock KM, Kebede S, Stevens KA, Prakasa KR, Shihab HM, et al. Pharmacologic and Mechanical Prophylaxis of Venous Thromboembolism Among Special Populations. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- Hewson JR, Neame PB, Kumar N, Ayrton A, Gregor P, Davis C, Shragge BW. Coagulopathy related to dilution and hypotension during massive transfusion. Crit Care Med. 1985;13(5):387–391.
- Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62(2):307–310.
- Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, Champion HR, Lawnick M, Farr W, Rodriguez S, et al. Causes of death in U.S. special operations forces in the global war on terrorism: 2001–2004. Ann Surg. 2007;245(6):986–991.
- Hess JR, Holcomb JB, Hoyt DB. Damage control resuscitation: the need for specific blood products to treat the coagulopathy of trauma. Transfusion. 2006;46(5):685–686.
- Biffl WL, Smith WR, Moore EE, Gonzalez RJ, Morgan SJ, Hennessey T, Offner PJ, Ray CE, Franciose RJ, Burch JM. Evolution of a multidisciplinary clinical pathway for the management of unstable patients with pelvic fractures. Ann Surg. 2001;233(6):843–850.
- Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, Cocanour CS, Balldin BC, McKinley BA. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62(1):112–119.
- Novak DJ, Bai Y, Cooke RK, Marques MB, Fontaine MJ, Gottschall JL, Carey PM, Scanlan RM, Fiebig EW, Shulman IA, et al. Making thawed universal donor plasma available rapidly for massively bleeding trauma patients: experience from the Pragmatic, Randomized Optimal Platelets and Plasma Ratios (PROPPR) trial. Transfusion. 2015;55(6):1331–1339.
- Callcut RA, Cotton BA, Muskat P, Fox EE, Wade CE, Holcomb JB, Schreiber MA, Rahbar MH, Cohen MJ, Knudson MM, et al. Defining when to initiate massive transfusion: a validation study of individual massive transfusion triggers in PROMMTT patients. J Trauma Acute Care Surg. 2013;74(1):59–65; 67–8; discussion 66–7.
- Cotton BA, Dossett LA, Au BK, Nunez TC, Robertson AM, Young PP. Room for (performance) improvement: provider-related factors associated with poor outcomes in massive transfusion. J Trauma. 2009;67(5):1004–1012.
- Wilson RF, Mammen E, Walt AJ. Eight years of experience with massive blood transfusions. J Trauma. 1971;11(4):275–285.
- Holcomb JB. Optimal use of blood products in severely injured trauma patients. Hematology Am Soc Hematol Educ Program. 2010;2010:465–469.
- Murad MH, Stubbs JR, Gandhi MJ, Wang AT, Paul A, Erwin PJ, Montori VM, Roback JD. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion. 2010;50(6):1370–1383.
- Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, Cothren CC, Biffl WL, Banerjee A, Sauaia A. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65(2):261–270.
- Scalea TM, Bochicchio KM, Lumpkins K, Hess JR, Dutton R, Pyle A, Bochicchio GV. Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Ann Surg. 2008;248(4):578–584.
- Holcomb JB, Zarzabal LA, Michalek JE, Kozar RA, Spinella PC, Perkins JG, Matijevic N, Dong JF, Pati S, Wade CE, et al. Increased platelet:RBC ratios are associated with improved survival after massive transfusion. J Trauma. 2011;71(2 Suppl 3):S318–S328.
- Pidcoke HF, Aden JK, Mora AG, Borgman MA, Spinella PC, Dubick MA, Blackbourne LH, Cap AP. Ten-year analysis of transfusion in Operation Iraqi Freedom and Operation Enduring Freedom: increased plasma and platelet use correlates with improved survival. J Trauma Acute Care Surg. 2012;73:S445–S452.
- del Junco DJ, Holcomb JB, Fox EE, Brasel KJ, Phelan HA, Bulger EM, Schreiber MA, Muskat P, Alarcon LH, Cohen MJ, et al. Resuscitate early with plasma and platelets or balance blood products gradually: findings from the PROMMTT study. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S24–S30.
- Ho AM, Dion PW, Yeung JH, Holcomb JB, Critchley LA, Ng CS, Karmakar MK, Cheung CW, Rainer TH. Prevalence of survivor bias in observational studies on fresh frozen plasma:erythrocyte ratios in trauma requiring massive transfusion. Anesthesiology. 2012;116(3):716–728.
- Kornblith LZ, Howard BM, Cheung CK, Dayter Y, Pandey S, Busch MP, Pati S, Callcut RA, Vilardi RF, Redick BJ, et al. The whole is greater than the sum of its parts: hemostatic profiles of whole blood variants. J Trauma Acute Care Surg. 2014;77(6):818–827.
- Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009;66(Suppl 4):S69–S76.
- Armand R, Hess JR. Treating coagulopathy in trauma patients. Transfus Med Rev. 2003;17(3):223–231.
- Kenet G, Walden R, Eldad A, Martinowitz U. Treatment of traumatic bleeding with recombinant factor VIIa. Lancet. 1999;354(9193):1879.
- Stein DM, Dutton RP, Hess JR, Scalea TM. Low-dose recombinant factor VIIa for trauma patients with coagulopathy. Injury. 2008;39(9):1054–1061.
- Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010;363(19):1791–1800.
- Cohen MJ. Towards hemostatic resuscitation: the changing understanding of acute traumatic biology, massive bleeding, and damage-control resuscitation. Surg Clin North Am. 2012;92(4):877–891, viii.
- Pusateri AE, Weiskopf RB, Bebarta V, Butler F, Cestero RF, Chaudry IH, Deal V, Dorlac WC, Gerhardt RT, Given MB, et al. Tranexamic acid and trauma: current status and knowledge gaps with recommended research priorities. Shock. 2013;39(2):121–126.
- Harvin JA, Peirce CA, Mims MM, Hudson JA, Podbielski JM, Wade CE, Holcomb JB, Cotton BA. The impact of tranexamic acid on mortality in injured patients with hyperfibrinolysis. J Trauma Acute Care Surg. 2015;78(5):905–909; discussion 909–11.
- CRASH-2 collaborators Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377(9771):1096–1101, 1101.e1–2.
- Rotondo MF, Cribari C, Smith RS. Resources for optimal care of the injured patient. Chicago, IL: American College of Surgeons; 2014.
- ACS TQIP guidelines, massive transfusion in trauma. Available at: https://www.facs.org/quality-programs/trauma/tqip/best-practice. Accessed May 7, 2016.
- National Institute for Health and Care Excellence (NICE) guidelines, major trauma, assessment and initial management. Available at: https://www.nice.org.uk/guidance/ng39. Accessed May 7, 2016.