Management of the open abdomen: A systematic review with meta-analysis and practice management guideline from the Eastern Association for the Surgery of Trauma
Published 2022
Citation: J Trauma, 93(3)::e110-e118, September 2022
Authors
Mahoney, Eric J. MD, FACS; Bugaev, Nikolay MD; Appelbaum, Rachel MD; Goldenberg-Sandau, Anna DO; Baltazar, Gerard A. DO, FACOS, FACS; Posluszny, Joseph MD; Dultz, Linda MD, MPH; Kartiko, Susan MD, PhD, FACS; Kasotakis, George MD, MPH, FACS, FCCM; Como, John MD; Klein, Eric MD, FACS
Advances in trauma care have improved survival after abdominal catastrophes.[1] Nonetheless, patients with open abdomens (OAs) are at risk of increased morbidity. Multiple techniques have been described to manage the OA, including temporary abdominal closure (e.g., the Bogota bag),[2] negative pressure wound therapy (NPWT),[3 ][4] and fascial traction systems [suture traction,[5] the ABRA system,[6] Wittmann Patch,[7] progressive partial fascial closure, and mesh-mediated fascial traction (MMFT).[8] Volume removal techniques[9] and complex abdominal reconstruction techniques including component separation[10]; bridging with biologic prostheses,[11 ][12] or placement of synthetic absorbable mesh and eventual skin grafting[13] have also been described. Despite the variety of options, the optimal treatment remains unclear.
In 2011, the Eastern Association for the Surgery of Trauma Practice Management Guidelines (PMG) Committee attempted to address this issue.[14 ][15] The authors concluded that “the populations are so heterogeneous” and that the “current literature remains contentious at best,” such that no recommendations could be provided. Since then, a significant body of evidence has emerged, and an updated systematic review and meta-analysis was deemed prudent. The goal of this article was to provide up-to-date recommendations regarding the optimal strategies for the OA after damage-control laparotomy (DCL).
Methods
Two population (P), intervention (I), comparator (C), and outcome (O) (PICO) questions were defined before the literature search:
PICO 1
In hemodynamically normal trauma and emergency general surgery (EGS) patients with OA after DCL in whom intra-abdominal pathology has been addressed and physiology normalized (P), should interventions to reduce visceral edema (diuresis, hypertonic saline, direct peritoneal resuscitation); (I) versus no interventions (C) be performed to help achieve primary myofascial closure during index admission, reduce ventral herniation after primary myofascial closure during index admission, reduce fascial dehiscence after primary myofascial closure, and reduce incidence of enterocutaneous/atmospheric fistula (ECF) and mortality (O)?
PICO 2
In hemodynamically normal trauma and EGS patients with OA after DCL in whom intra-abdominal pathology has been addressed (P), should a fascial traction system be used (I) versus no traction systems (C) to help achieve primary myofascial closure during index admission, reduce ventral herniation after primary myofascial closure during index admission, reduce fascial dehiscence after primary myofascial closure, and reduce incidence of ECF and mortality (O)?
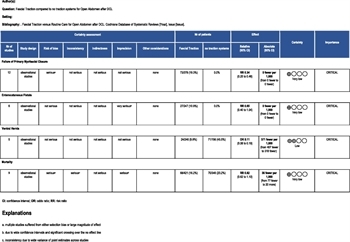
Selection of Outcome Measures
Clinically relevant outcomes were identified and rated on a scale of 1 to 9. Outcomes that averaged 7 to 9 were considered critical and were used for analysis. These included mortality, failure of primary myofascial closure during index admission, ventral herniation after primary myofascial closure during index admission, fascial dehiscence after primary myofascial closure, and ECF. Initially, 3,878 abstracts were identified, of which 19 articles met the inclusion criteria (PRISMA, Supplement Digital Content A, https://links.lww.com/TA/C524).
Identification of References
A professional medical librarian (J.R.) performed searches of citations in , Embase, Cochrane Library, Web of Science and Ovid Medline. The MeSH search terms included: PICO 1: laparotomy, volume removal, goal-directed diuresis, hypertonic saline solution, renal replacement therapy, dialysis; PICO 2: fascia, traction, Wittmann Patch, abdominal reapproximation anchor, ABRA system, progressive partial fascial closure, mesh-mediated fascial mobilization, Vacuum-Assisted Wound Closure and Mesh-Mediated Fascial Traction (VAWCM) technique. Abstract reviews, full-text reviews, and data extraction were performed in duplicate utilizing Covidence (www.covidence.org).
Randomized control trials, observational studies, and retrospective reviews with comparison groups in adults (age, ≥18 years) in English or English-translated articles (1950 to present) were included in the analyses. Case series, case reports, review articles, meta-analyses, and non-peer reviewed open access articles were excluded. Article reference lists were reviewed to ensure that no relevant articles were overlooked.
Data Extraction and Methodology
Each abstract and full text was assigned to two working group members to determine if the article met inclusion criteria. A third member (E.M.) adjudicated any difference in opinion. Data were extracted onto a standardized data collection sheet and collated into a master file.
The meta-analysis was conducted with random effects modeling and forest plots were generated using Review Manager (RevMan5; Version 5.3; Cochrane Collaboration, Oxford). Of note, it was determined the RevMan5 inherently favors outcomes with less incidence (e.g., mortality). Therefore, the outcome for fascial closure was analyzed as “less failure of primary myofascial closure” rather than “group with greater fascial closure.” The degrees of heterogeneity (I[2]) were calculated between study populations and were defined as low (I[2] < 50%) or high (I[2] > 50%).
“Physiology normalized” was defined as the time after acute resuscitation when shock has been corrected and the end-organ perfusion has been restored, either off pressor medications or on a minimal, stable dose. “Intra-abdominal pathology has been addressed” was defined as the time after infectious source control has been established, and no further resection of intra-abdominal or abdominal wall tissue is anticipated. “Skin only” closure was considered a ventral hernia. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology was utilized to determine the impact of selected interventions and to assess the level of evidence.[16] The GRADE evidence profile table was created utilizing online software (gdt.gradepro.org).
The quality of the available evidence was assessed as high, moderate, low, or very low, based on study design, selection bias, inconsistency, indirectness, imprecision, magnitude of effect, and plausible confounding variables. The quality of evidence was graded up or down based on these principles. Recommendation consensus was reached by blinded voting and the group articulated a strong recommendation as “we recommend,” whereas a weak recommendation was listed as “we conditionally recommend.”
Fascial Traction Systems
Progressive fascial closure and suture traction, as described by Cothren and colleagues,[5] is a technique wherein the patient with OA is returned to the operating room at a set interval (usually every 48 hours). During each procedure, fascial sutures are placed in interrupted fashion until tension develops along the incision. The remaining OA is covered with a protective temporary closure. The ABRA system utilizes a series of midline-crossing elastomers that are inserted perpendicular to the fascia and are tightened daily to apply constant fascial tension.[6] The Wittmann Patch utilizes two sheets of complementary material: one hook sheet and one loop sheet to provide adherence.[7] Each sheet is secured to one side of the OA with transfascial sutures. The sheets are pulled taut and pressed together sequentially until the fascia is reapproximated. In MMFT, a polypropylene mesh is sewn circumferentially to the fascia of the OA.[8] The mesh is “pinched” daily to determine if laxity has developed. If laxity is identified, the mesh is sutured to maintain fascial traction. A negative pressure therapy dressing is applied often, creating a VAWCM technique.
Results
PICO Question 1
Qualitative Analysis
Diuresis
Two studies evaluating the use of diuretic therapy after DCL met our criteria. Webb et al.[9] performed a single institution, retrospective review of patients with an OA more than 24 hours who received furosemide compared with those who did not. The selection criteria detailing the choice of patients to receive furosemide therapy was poorly described. The article did not offer a treatment protocol nor the average amount of medication each patients received. Furthermore, the authors did not document volume status nor if a negative fluid balance was achieved after furosemide infusion. The study by Tian and colleagues[17] was a prospective, protocolized design. All patients in the treatment group received 20% Albumin intravenously, followed by 20 mg of Torsemide IV daily for 7 days. This study had several significant limitations as well. Group assignment was based on patients or the health care proxy preference and not by randomization. Moreover, the presence or development of a fistula before abdominal closure excluded the patients from analysis. The use of the diuretic was in conjunction with a treatment protocol that included VAWCM technique and continuous peritoneal instillation of saline, thus making it difficult to ascertain the contribution that diuresis made to patient recovery. Furthermore, 11 of the 16 patients receiving diuretic therapy also underwent dialysis. Finally, the closure technique for all these patients was by component separation, which may not be the case with the study by Webb and colleagues.
Hypertonic Saline
Two studies evaluating the use of hypertonic saline (HTS) to improve the rates of primary myofascial closure met our criteria. The study by Harvin et al.[18] was limited by its retrospective, observational design, and lack of protocolization. The use of HTS was at the discretion of the attending surgeon, which may have introduced bias. The HTS study by Loftus et al.[19] was also a retrospective study in which patients who were treated with HTS as part of a treatment protocol were compared with matched historical controls. Unfortunately, the extensive exclusion criteria, which excluded patients with acute kidney injury Kidney Disease Improving Global Outcomes stage 2 or greater, chronic kidney disease stage 3 or greater, pH less than 7.10, or cirrhosis, created a select treatment group and makes any comparison with the group described by Harvin and colleagues difficult.
Direct Peritoneal Resuscitation
The three studies by Smith and colleagues[20–22] demonstrate a methodical commitment to understand the benefits of direct peritoneal resuscitation (DPR). The studies develop from a retrospective case-matched study of patients undergoing DCL for hemorrhagic shock, progress to a propensity-matched study of peritoneal resuscitation in all EGS patients who required DCL, and then culminate in a prospective randomized controlled trial in a similar cohort. Although these compelling findings demonstrate improved primary myofascial closure rates in patients receiving DPR, one must proceed with some hesitation until additional independent studies provide corroboration.
Quantitative Analysis
Diuresis
In their retrospective review, Webb et al.[9] did not find improved primary myofascial closure rates with the use of a furosemide infusion. Conversely, Tian and colleagues[17] were able to demonstrate a higher incidence of primary myofascial closure when therapeutic diuresis was included. There was no difference in mortality between the groups in either study. Neither study addressed the other outcomes selected for our analysis.
Hypertonic Saline
Both studies employed 3% HTS at 30 mL/h. as the maintenance fluid after DCL. Harvin et al.[18] demonstrated that the patients in the control group received significantly more fluid in the 24-hour, 48-hour, and 72-hour timepoints compared with those receiving HTS. This difference translated into a higher rate of failure to achieve primary myofascial closure in the control group versus the HTS group (24% vs. 4%) at discharge; unfortunately, this did not meet statistical significance. Thirty-day mortality was the same in both groups. Similarly, Loftus et al.[19] initiated 3% HTS in patients with OA (plus bolus isotonic fluid to achieve euvolemia). Progressive fascial closure was performed at each staged repeat laparotomy. These researchers found that patients treated with HTS received less total fluid during the first 48 hours after DCL compared to standard therapy, yet the total fluids received between the two groups at 96-hour and 7-day timepoints were the same. Nonetheless, the authors were able to demonstrate improved primary myofascial closure rates in the HTS group compared with the controls without a statistical difference in the incidence of fascial dehiscence nor mortality.
Direct Peritoneal Resuscitation
Three studies evaluating the use of DPR were identified. All the studies originated from Smith and colleagues.[20–22] The first study demonstrated that treatment with DPR was associated with a statistical improvement in primary myofascial closure compared with controls.[20] This difference was lost when DPR was compared with controls who were treated with Wittmann Patch. Direct peritoneal resuscitation was associated with a statistically significant decrease in the incidence of hernia development without any statistically significant difference in the incidence of ECF nor mortality. In their second and third studies, Smith et al. were able to demonstrate that patients treated with DPR had a higher incidence of primary myofascial closure compared with controls (Smith et al.[21] primary myofascial closure: 68% vs. 43%, p = 0.03; Smith et al.[22] primary myofascial closure: 83% vs. 67%, p = 0.005). Mortality and the incidence of ECF were not statistically difference between DPR and control groups in either study.

Figure 1: Forest plot, failure of primary myofascial closure with techniques to reduce visceral edema.
The working group determined that it was important to include a comparison of these treatment techniques. Although the techniques varied, they shared the intent of reducing visceral edema to improve fascial closure. Overall, only four studies were suitable for meta-analysis.[9 ][17–19] The studies by Smith and colleagues demonstrated significant overlap in the enrollment timepoints for each of the studies (Smith et al.[20]: January 2005 to December 2008; Smith et al.[21]: January 2008 to December 2012; Smith et al.[22]: January 2011 to December 2015). Thus, we could not include these studies since it seems likely that patients from the overlapping timepoints may be represented in more than one study. Only one study demonstrated a clear improvement in primary myofascial closure with the reduction of visceral edema.[17] There were no difference in in-hospital mortality among any of the studies. None of the studies evaluated ECF, fascial dehiscence or recurrent hernia. In all, 124 patients who underwent a technique to reduce visceral edema and 257 patients with routine care were included in the meta-analysis. This analysis demonstrated that the addition of a technique to reduce visceral edema may lessen the failure of primary myofascial closure in patients with an OA (relative risk [RR], 0.52; 95% confidence interval [CI], 0.33–0.81; Fig. 1). However, the findings are very limited based on the high heterogeneity (as demonstrated by an I[2] of 71%) and the small number of studies.
Grading the Evidence
Methodological variations of the study designs limit direct comparisons. Only two outcomes of interest (primary closure and mortality) were evaluated in the studies. Based on the small number of studies, and the limitations identified in the qualitative analysis, the quality of the evidence was considered very low. A GRADE evidence profile table was deemed fruitless because of the lack of outcomes available and high heterogeneity.
Recommendations
We are unable to make any recommendations regarding the use of techniques to remove visceral edema in hemodynamically normal trauma and emergency general surgery patients with OAs after DCLs.
PICO Question 2
Qualitative Analysis
Most studies were retrospective studies[5–7 ][23–27] and only four of the studies[8 ][28–30] were prospective randomized trials. Nine of the studies[5–7 ][23–27 ][29] did reveal a large magnitude of effect in the intervention groups compared with the control groups. Unfortunately, blinding was not possible in any of the studies and may have affected the outcomes. There appeared to be selection bias in four studies,[6 ][24 ][26 ][28] imprecision in two studies[8 ][23] and confounding variables in two studies.[5 ][28] Sample size plagued many studies, thus limiting the ability to draw firm conclusions on any of the outcomes.[8 ][24 ][27 ][28 ][30] Please see the GRADE Evidence Profile (GRADEpro, Table 1).
Quantitative Analysis
Primary myofascial closure during index admission
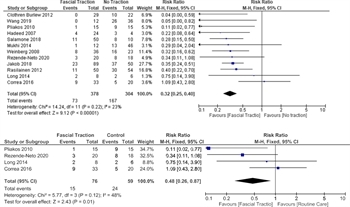
Figure 2: Forest plot, failure of primary myofascial closure, fascial traction vs. routine care; (A) All studies, (B) Randomized, controlled trials only.
Twelve studies were identified that addressed the outcome measure of fascial traction: four were randomized controlled trials[8 ][28–30] and the remainder were retrospective observational studies.[5–7 ][23–27] Three evaluated MMFT[8 ][23 ][25] as the study group, two Wittmann patch,[7 ][27] three ABRA plus NPWT,[6 ][24 ][28] two suture fascial traction,[5 ][29] one allowed either vessel loop or MMFT as the mode of fascial traction,[26] and one evaluated a novel fascial traction device.[30] Overall, the studies included 378 patients in the fascial traction group, and 304 patients in the control group. Most compared the fascial traction group against NPWT alone,[6 ][8 ][23 ][24 ][28–30] one versus traction plus NPWT,[5] one versus nontraction mesh,[26] and three against various techniques (e.g., Bogota bag, mesh).[7 ][25 ][27] Data from all included studies were suitable for analysis. Three studies did not show a statistically significant improvement[8 ][24 ][28] and nine demonstrated improvement favoring fascial traction.[5–7 ][23 ][25–27 ][29 ][30] When all of the studies were included, the populations were determined to have a low degree of heterogeneity based on an I[2] value of 23% and, fascial traction was favored over non-fascial traction (RR, 0.34; (95% CI, 0.25–0.46). When only randomized, controlled trials were included, fascial traction was still favored (RR, 0.48; 95% CI, 0.26–0.87] but with higher heterogeneity (I[2] = 48%; Fig. 2A and B).
Two studies found that the patients with fascial traction strategies had abdominal closure 4 days sooner[8 ][29] and one study reported 43 days sooner.[27] Conversely, two studies found that patients with fascial traction devices had more days with OAs despite less synthetic mesh placement and greater fascial closure rates.[6 ][7] Rasilainen and colleagues[25] reported no difference in the mean number of OA days. The remaining studies did not comment on the time to closure between the groups.[5 ][23 ][24 ][26 ][28 ][30]
Enterocutaneous and Enteroatmospheric Fistulas
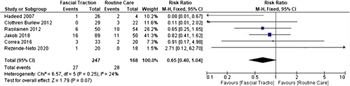
Figure 3: Forest plot, incidence of enterocutaneous and enteroatmospheric fistula, fascial traction vs. routine care.
Six studies were suitable for analysis.[5 ][8 ][25–27 ][30] All studies identified the ECF during the index hospitalization. Only one study reported improvement in ECF rates with fascial traction, Wittmann patch, versus Bogota bag placement (1/24 patients with WP developed ECF, vs. 2/4 in Bogota group).[27] Overall, 247 patients with fascial traction versus 168 in control were included in the meta-analysis. These groups were determined to have a low degree of heterogeneity based on an I[2] value of 24%. This analysis demonstrated no statistically significant difference in the incidence of ECF rates (RR, 0.65; 95% CI, 0.40–1.04; Fig. 3).
Fascial Dehiscence
Two studies addressed this outcome in their analysis.[8 ][30] Correa and colleagues[8] identified a fascial dehiscence rate of 15% of patients treated with NPWT alone compared to 6.3% when MMFT was added. These values did not reach statistical significance. Rezende-Neto and Camilotti[30] reported that there were no cases of fascial dehiscence in patients treated with a fascial reapproximation device in addition to NPWT. Unfortunately, the authors did not report the incidence of fascial dehiscence in the control group.
Ventral Herniation
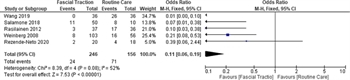
Five studies addressed ventral hernia.[6 ][7 ][23 ][25 ][30] The presence of a hernia was determined at the time of discharge in all studies, and included overt hernia, skin only closure, and skin graft onto the OA with planned ventral hernia repair in the future. Two studies evaluated MMFT[23 ][25] versus NPWT without fascial traction, one evaluated the ABRA system plus NPWT versus NPWT alone;[6] one evaluated a novel fascial traction device versus NPWT,[30] and one evaluated the Wittmann patch plus NPWT versus various other techniques (e.g., Bogota bag, NPWT, mesh).[7] The meta-analysis included 246 patients in the study group versus 156 in the control group and favored facial traction (odds ratio, 0.11; 95% CI, 0.06–0.19; Figure 4). Unfortunately, these results are limited by the high heterogeneity identified between study populations (I[2] = 52%).
Mortality
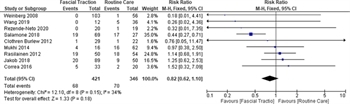
Mortality, which was based upon death before discharge, was addressed in nine studies.[5–8 ][23–26 ][30] The meta-analysis included 421 patients in the study group and 346 in the control group. Only one study demonstrated a survival benefit for fascial traction.[23] The remainder of the studies did not demonstrate any difference in mortality when comparing fascial traction to no fascial traction (RR, 0.82; 95% CI, 0.62–1.10; Fig. 5). The I[2] value of 34% suggests low heterogeneity between the studies.
Grading the Evidence
Based on the retrospective nature of the many of the studies, the small number of studies, and the limitations identified in the qualitative analysis, the quality of the evidence was considered very low.
Recommendations
We conditionally recommend the use of a fascial traction system in hemodynamically normal trauma and emergency general surgery patients with OA after DCL in whom intra-abdominal pathology has been addressed.
Discussion
Using These Guidelines in Clinical Practice
At times, primary myofascial closure after OA is not possible due to visceral edema and patient physiology. The optimal treatment strategy in this situation has been elusive. Our findings suggest that fascial traction systems improve the rate of primary myofascial closure over routine care without any worsening in mortality or ECF formation.
In the time since the Eastern Association for the Surgery of Trauma guidelines for the management of the OA were published, multiple systematic reviews have been performed. The American Association for the Surgery of Trauma sponsored a multi-institutional study to identify the natural history of the OA at 14 Level I trauma centers.[31] Most facilities (94%) used NPWT alone. Of the 572 patients, 338 had definitive primary fascial closure, and 138 were treated with alternative therapies such as split-thickness skin graft, synthetic mesh, biological matrices, or component separation. This study was limited, however, as an observational study. Importantly, there was no mention of the use of fascial traction.
Quyn et al.[32] performed a systematic review of TAC over 30 years to describe the evolution of techniques and to determine closure rates. Wittmann patch plus NPWT was associate with the highest closure rate (77.8%), the lowest mortality rate (15.7%), and the lowest complication rate (ECF 2.8%, abscess 2.4%). Dynamic retention sutures had the next best results with primary myofascial closure rates of 72%; ECF, 10%; abscess, 2%; and mortality, 18%. These findings support our view that fascial tractions systems improve closure rates with minimal morbidity and mortality.
Atema et al.[33] performed a systematic review of publications addressing the treatment of the OA in patients with peritonitis of nontraumatic origin. The findings suggested that the highest fascial closure rate was seen for NPWT with fascial traction (73.1%) and dynamic retention sutures (73.6%). Moreover, the lowest rate of ECF formation was found in NPWT with fascial traction. The authors postulated that NPWT with continuous fascial traction is superior to NPWT alone and other OA techniques. Based on our findings, we concur with these conclusions.
In 2016, Sharrock et al.[34] performed a meta-analysis comparing the outcomes of the differing techniques. The authors were unable to recommend one technique over another, citing “study heterogeneity and poverty of outcome reporting.” Also, that year, the International Consensus Conference was unable to provide recommendations as to the best method to obtain primary myofascial closure.[35] We think that we have overcome the barriers faced by Sharrock and colleagues by limiting our analysis to only studies with comparison groups and by excluding case series. During our search, we found 20 case series and review articles that evaluated the treatment of the OA with fascial traction methodology (see Supplement B, https://links.lww.com/TA/C525). While these results are mentioned for discussion purposes only, with few exceptions, these studies strongly support our findings that fascial traction provide higher primary myofascial closure rates after OA.
Our findings are different from those of Bee et al.[36] in their randomized controlled trial of OA treated with Vicryl mesh versus NPWT. The authors did not find any difference in the rate of successful closure between these two techniques. However, the method of mesh use did not address the technique of mesh with fascial traction. While the mesh was resutured twice daily if it was found to be loose, the authors did not appear to be actively creating fascial traction. Therefore, the difference between this study and the findings of others appear related to fascial traction improving the incidence of primary myofascial closure.
Our study has several limitations. We did not differentiate outcomes based on the clinical indication for DCL. Previous authors have demonstrated that trauma patients have a higher rate of closure than EGS patients.[37 ][38] Furthermore, several of the studies included were performed before damage-control resuscitation was widely practiced. Therefore, our findings may not reflect the impact that this strategy has on improving success rates of primary myofascial closure. Nonetheless, treating a recalcitrant OA remains a challenge in either cohort, and we think that our results provide important considerations. Moreover, we did not attempt to address the non-mechanical benefits that adjuvant therapies may provide. Smith and colleagues[22] have demonstrated that patients treated with DPR had lower levels of TNF-α and IL-6, which may be associated with less systemic inflammation compared with routine care. A better understanding of this complex interplay between adjuvant therapies and patient outcomes would advance trauma care greatly. Also, the quality of evidence in both PICO questions was considered to be very low. Additional well-designed studies evaluating treatments options for patients with recalcitrant OAs are sorely needed.
Conclusion
Our findings reveal that fascial traction systems improve the rate of primary myofascial closure over routine care without worsening mortality or ECF formation. Therefore, we conditionally recommend their use. We are unable to make any recommendations regarding the use of techniques to reduce visceral edema in patients with OA. Our findings are limited because of the small number of quality studies. Additional studies evaluating different treatment options and complex abdominal wall reconstruction techniques for the OA are urgently needed.
Authorship
E.J.M. contributed in the study design, chart review, data analysis, article preparation. G.K. contributed in the study design, data analysis, article preparation. J.C. contributed in the study design, article preparation. N.B. contributed in the study design, chart review, data analysis, article preparation. R.A. contributed in the study design, chart review, article preparation. A.G.-S. contributed in the study design, chart review, article preparation. G.A.B. contributed in the study design, chart review, article preparation. L.D. contributed in the study design, chart review, article preparation. J.P., study design, chart review, article preparation. S.K. contributed in the study design, chart review, article preparation. E.K. contributed in the study design, chart review, article preparation.
Acknowledgements
We would like to acknowledge Judy Rabinowitz, Medical Librarian, Hirsh Health Sciences Library, Tufts Medical School, for her invaluable assistance and meticulous literature search. Janis Breeze, MPH, Associate Director, BERD Center, Tufts Clinical and Translational Science Institute aided with statistical analysis (NIH CTSA award number UL1TR002544).
Disclosure
The authors declare no conflicts of interest.
References
- Diaz JJ Jr., Cullinane DC, Dutton WD, Jerome R, Bagdonas R, Bilaniuk JW, et al. The management of the open abdomen in trauma and emergency general surgery: part 1—damage control. J Trauma. 2010;68(6):1425–1438.
- Fernandez L, Norwood S, Roettger R, Wilkins HE. Temporary intravenous bag silo closure in severe abdominal trauma. J Trauma. 1996;40(2):258–260.
- Barker DE, Kaufman HJ, Smith LA, Ciraulo DL, Richart CL, Burns RP. Vacuum pack technique of temporary abdominal closure: a 7-year experience with 112 patients. J Trauma. 2000;48(2):201–206; discussion 206-207.
- Suliburk JW, Ware DN, Balogh Z, McKinley BA, Cocanour CS, Kozar RA, et al. Vacuum-assisted wound closure achieves early fascial closure of open abdomens after severe trauma. J Trauma. 2003;55(6):1155–1160; discussion 1160-1161.
- Cothren CC, Moore EE, Johnson JL, Moore JB, Burch JM. One hundred percent fascial approximation with sequential abdominal closure of the open abdomen. Am J Surg. 2006;192(2):238–242.
- Wang Y, Alnumay A, Paradis T, Beckett A, Fata P, Khwaja K, et al. Management of open abdomen after trauma laparotomy: a comparative analysis of dynamic fascial traction and negative pressure wound therapy systems. World J Surg. 2019;43(12):3044–3050.
- Weinberg JA, George RL, Griffin RL, Stewart AH, Reiff DA, Kerby JD, et al. Closing the open abdomen: improved success with Wittmann patch staged abdominal closure. J Trauma. 2008;65(2):345–348.
- Correa JC, Mejía DA, Duque N, J MM, Uribe CM. Managing the open abdomen: negative pressure closure versus mesh-mediated fascial traction closure: a randomized trial. Hernia. 2016;20(2):221–229.
- Webb LH, Patel MB, Dortch MJ, Miller RS, Gunter OL, Collier BR. Use of a furosemide drip does not improve earlier primary fascial closure in the open abdomen. J Emerg Trauma Shock. 2012;5(2):126–130.
- Poulakidas S, Kowal-Vern A. Component separation technique for abdominal wall reconstruction in burn patients with decompressive laparotomies. J Trauma. 2009;67(6):1435–1438.
- Velmahos GC, Demetriades D, Mahoney E, Burke P, Davis K, Larentzakis A, et al. The worst-case scenario: bridging repair with a biologic mesh in high-risk patients with very large abdominal wall hernias—a prospective multicenter study. Surgery. 2021;169(2):318–324.
- de Moya MA, Dunham M, Inaba K, Bahouth H, Alam HB, Sultan B, et al. Long-term outcome of acellular dermal matrix when used for large traumatic open abdomen. J Trauma. 2008;65(2):349–353.
- Jernigan TW, Fabian TC, Croce MA, Moore N, Pritchard FE, Minard G, et al. Staged management of giant abdominal wall defects: acute and long-term results. Ann Surg. 2003;238(3):349–355; discussion 355-357.
- Diaz JJ Jr., Dutton WD, Ott MM, Cullinane DC, Alouidor R, Armen SB, et al. Eastern Association for the Surgery of Trauma: a review of the management of the open abdomen—part 2 “Management of the open abdomen”. J Trauma. 2011;71(2):502–512.
- Diaz JJ Jr., Cullinane DC, Khwaja KA, Tyson GH, Ott M, Jerome R, et al. Eastern Association for the Surgery of Trauma: management of the open abdomen, part III—review of abdominal wall reconstruction. J Trauma Acute Care Surg. 2013;75(3):376–386.
- Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, et al. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S283–S287.
- Tian W, Huang Q, Yao Z, Huang M, Yang F, Zhao Y, et al. A preliminary prospective study of patients who underwent vacuum-assisted and mesh-mediated fascial traction techniques for open abdomen management with negative fluid therapy: an observational study. Medicine (Baltimore). 2019;98(35):e16617.
- Harvin JA, Mims MM, Duchesne JC, Cox CS Jr., Wade CE, Holcomb JB, et al. Chasing 100%: the use of hypertonic saline to improve early, primary fascial closure after damage control laparotomy. J Trauma Acute Care Surg. 2013;74(2):426–430; discussion 431-432.
- Loftus TJ, Efron PA, Bala TM, Rosenthal MD, Croft CA, Walters MS, et al. The impact of standardized protocol implementation for surgical damage control and temporary abdominal closure after emergent laparotomy. J Trauma Acute Care Surg. 2019;86(4):670–678.
- Smith JW, Garrison RN, Matheson PJ, Franklin GA, Harbrecht BG, Richardson JD. Direct peritoneal resuscitation accelerates primary abdominal wall closure after damage control surgery. J Am Coll Surg. 2010;210(5):658–664, 664-667.
- Smith JW, Neal Garrison R, Matheson PJ, Harbrecht BG, Benns MV, Franklin GA, et al. Adjunctive treatment of abdominal catastrophes and sepsis with direct peritoneal resuscitation: indications for use in acute care surgery. J Trauma Acute Care Surg. 2014;77(3):393–398; discussion 398-399.
- Smith JW, Matheson PJ, Franklin GA, Harbrecht BG, Richardson JD, Garrison RN. Randomized controlled trial evaluating the efficacy of peritoneal resuscitation in the management of trauma patients undergoing damage control surgery. J Am Coll Surg. 2017;224(4):396–404.
- Salamone G, Licari L, Guercio G, Comelli A, Mangiapane M, Falco N, et al. Vacuum-assisted wound closure with mesh-mediated fascial traction achieves better outcomes than vacuum-assisted wound closure alone: a comparative study. World J Surg. 2018;42(6):1679–1686.
- Mukhi AN, Minor S. Management of the open abdomen using combination therapy with ABRA and ABThera systems. Can J Surg. 2014;57(5):314–319.
- Rasilainen SK, Mentula PJ, Leppäniemi AK. Vacuum and mesh-mediated fascial traction for primary closure of the open abdomen in critically ill surgical patients. Br J Surg. 2012;99(12):1725–1732.
- Jakob MO, Schwarz C, Haltmeier T, Zindel J, Pinworasarn T, Candinas D, et al. Mesh-augmented versus direct abdominal closure in patients undergoing open abdomen treatment. Hernia. 2018;22(5):785–792.
- Hadeed JG, Staman GW, Sariol HS, Kumar S, Ross SE. Delayed primary closure in damage control laparotomy: the value of the Wittmann patch. Am Surg. 2007;73(1):10–12.
- Long KL, Hamilton DA, Davenport DL, Bernard AC, Kearney PA, Chang PK. A prospective, controlled evaluation of the abdominal reapproximation anchor abdominal wall closure system in combination with VAC therapy compared with VAC alone in the management of an open abdomen. Am Surg. 2014;80(6):567–571.
- Pliakos I, Papavramidis TS, Mihalopoulos N, Koulouris H, Kesisoglou I, Sapalidis K, et al. Vacuum-assisted closure in severe abdominal sepsis with or without retention sutured sequential fascial closure: a clinical trial. Surgery. 2010;148(5):947–953.
- Rezende-Neto JB, Camilotti BG. New non-invasive device to promote primary closure of the fascia and prevent loss of domain in the open abdomen: a pilot study. Trauma Surg Acute Care Open. 2020;5(1):e000523.
- Dubose JJ, Scalea TM, Holcomb JB, Shrestha B, Okoye O, Inaba K, et al. Open abdominal management after damage-control laparotomy for trauma: a prospective observational American Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg. 2013;74(1):113–120; discussion 1120-1122.
- Quyn AJ, Johnston C, Hall D, Chambers A, Arapova N, Ogston S, et al. The open abdomen and temporary abdominal closure systems—historical evolution and systematic review. Color Dis. 2012;14(8):e429–e438.
- Atema JJ, Gans SL, Boermeester MA. Systematic review and meta-analysis of the open abdomen and temporary abdominal closure techniques in non-trauma patients. World J Surg. 2015;39(4):912–925.
- Sharrock AE, Barker T, Yuen HM, Rickard R, Tai N. Management and closure of the open abdomen after damage control laparotomy for trauma. A systematic review and meta-analysis. Injury. 2016;47(2):296–306.
- Chiara O, Cimbanassi S, Biffl W, Leppaniemi A, Henry S, Scalea TM, et al. International consensus conference on open abdomen in trauma. J Trauma Acute Care Surg. 2016;80(1):173–183.
- Bee TK, Croce MA, Magnotti LJ, Zarzaur BL, Maish GO 3rd, Minard G, et al. Temporary abdominal closure techniques: a prospective randomized trial comparing polyglactin 910 mesh and vacuum-assisted closure. J Trauma. 2008;65(2):337–342; discussion 342-344.
- Tsuei BJ, Skinner JC, Bernard AC, Kearney PA, Boulanger BR. The open peritoneal cavity: etiology correlates with the likelihood of fascial closure. Am Surg. 2004;70(7):652–656.
- Loftus TJ, Jordan JR, Croft CA, Smith RS, Efron PA, Mohr AM, et al. Temporary abdominal closure for trauma and intra-abdominal sepsis: different patients, different outcomes. J Trauma Acute Care Surg. 2017;82(2):345–350.