Monitoring Modalities, Assessment of Volume Status, and Endpoints of Resuscitation
Replaces: Resuscitation Endpoints
Published 2018
Citation: J Trauma. 84(1):37–49, January 2018
Authors
Plurad, David S. MD; Chiu, William MD; Raja, Ali S. MD; Galvagno, Samuel M. PhD; Khan, Uzer MD; Kim, Dennis Y. MD; Tisherman, Samuel A. MD; Ward, Jeremy MD; Hamill, Mark E. MD; Bennett, Vicki MSN; Williams, Brian MD; Robinson, Bryce MD
Author Information
From the Department of Surgery (D.S.P., D.Y.K.), Harbor-UCLA Medical Center, David Geffen School of Medicine at UCLA, Torrance, California; Department of Surgery (W.C., S.A.T.), University of Maryland School of Medicine, Baltimore, Maryland; Department of Emergency Medicine (A.S.R.), Harvard School of Medicine, Boston, Massachusetts; Department of Anesthesiology (S.M.G.), University of Maryland School of Medicine, Baltimore, Maryland; Department of Surgery (U.K.), West Virginia University School of Medicine, Morgantown, West Virginia; Department of Surgery (J.W.), Baylor College of Medicine, Houston, Texas; Department of Surgery (M.A.H.), Carilion Clinic, Roanoke, Virginia; Banner Health (V.B.), Phoenix, Arizona; Department of Surgery (B.W.), University of Texas Southwestern, Dallas, Texas; and Department of Surgery (B.R.), University of Washington, Seattle, Washington.
Submitted: July 21, 2017, Revised: September 15, 2017, Accepted: October 2, 2017, Published online: October 11, 2017.
Preliminary data from this article were presented as a podium talk/status update during the Practice Management Guideline session at the 30th Annual Scientific Assembly of the Eastern Association for the Surgery of Trauma, January 10–14, 2017, Hollywood, FL.
Address for reprints: David S. Plurad, MD, Director, Trauma Services, Riverside Community Hospital, 4445 Magnolia Ave., Riverside, CA 92501; email: dsplurad@yahoo.com.
Overview
Liberal fluid administration can be associated with pulmonary dysfunction, organ failures, coagulopathy, and infectious complications.[1] Evidence neither supports central venous pressure (CVP) measurements,[2][3] nor the necessity for the pulmonary artery catheter to safely guide resuscitation.[4–6] There has been a shift to less invasive dynamic measures to monitor fluid status.[7–9] However, many of these modalities have not undergone comparison.[10] Administering a “fluid challenge” remains variable and frequently ill-advised.[11] Guidelines for early goal-directed therapy in sepsis call for frequent fluid assessments but do not specify methodology.[12] There is a need for tools to guide resuscitations. Using the “Grading of Recommendations Assessment, Development and Evaluation” (GRADE) process,[13] we performed a systematic review to define the role of focused ultrasound and Arterial Pulse Waveform Analysis (APWA) for surgical patients in shock.
Focused Ultrasound
Focused ultrasound is common in critical care.[14][15] It is used for risk stratification,[16] shock in the emergency department (ED),[17] treatment of sepsis[18] and trauma resuscitation.[19][20] Variations include, limited transthoracic echocardiogram (“LTTE”),[21] rapid ultrasound in shock-velocity time integral,[22] and Bedside Echocardiographic Assessment in Trauma/Critical Care.[23] In all variants,[24] the test is clinician performed for a specific problem, with a limited number of potential diagnoses, and can include noncardiac images. Expert reviews have recommended focused ultrasound to monitor resuscitation in various shock states.[15][24][25] However, other technologies exist,[26] previous strong recommendations are not specific to surgical resuscitations and are based on variable data. The optimal method for hemodynamic monitoring remains elusive[24] despite growing acceptance of focused ultrasound. Further, a large international evidence based review urged caution for the use of focused ultrasound to predict fluid responsiveness despite advocating for wider use.[14]
Arterial Pulse Waveform Analysis
Wesseling et al.[27] described a method to predict aortic flow, stroke volume variation (SVV) and, therefore, fluid responsiveness, using APWA. The Vigileo, FloTrac device (Edwards Lifesciences, Irvine, CA) utilizes algorithms to determine SVV, from standard deviations of the pulse pressure. The Pulse Contour Cardiac Output system (“PiCCO”; Pulsion SG, Munich), renders cardiac output (CO) by measuring the area of the systolic waveform and aortic impedance. This system also provides for calibration of CO via transpulmonary thermodilution. The LiDCO device (LiDCO Ltd, Cambridge) renders CO derived from pulse power analysis with lithium indicator method calibration. With an increasing permeation of these devices, clinicians require an awareness of their indications and limitations.[26]
Six population [P], intervention [I], comparator [C], and outcome [O] (PICO) questions[13] are addressed in this guideline:
PICO question 1:
In surgical patients being evaluated for shock [P], should a protocol that includes focused ultrasound [I] be utilized versus a standard protocol [C] to predict fluid responsiveness [O]?
PICO question 2:
In surgical patients being resuscitated from shock [P], should a protocol that includes focused ultrasound [I] be utilized versus a standard protocol [I] to reduce organ failures or complications [O]?
PICO question 3:
In surgical patients being resuscitated from shock [P], should a protocol that includes focused ultrasound [I] be utilized versus a standard protocol [C] to reduce mortality [O]?
PICO question 4:
In surgical patients being evaluated for shock [P], should a protocol that includes APWA [I] be utilized versus a standard protocol [C] to predict fluid responsiveness [O]?
PICO question 5:
In surgical patients being resuscitated from shock [P], should a protocol that includes APWA [I] be utilized versus a standard protocol [C] to reduce organ failures or complications [O]?
PICO question 6:
In surgical patients being resuscitated from shock [P], should a protocol that includes APWA [I] be utilized versus a standard protocol [C] to reduce mortality [O]?
Inclusion and Exclusion Criteria
Study Types
We included prospective randomized trials, case control studies, prospective observational studies, retrospective observational trials, and cohort studies with comparator groups.
Participant and Setting Types (Population, P)
We included adult surgical patients being evaluated for shock. This included hemodynamic instability or other indications for which fluid administration was considered. We also included studies of nonsurgical populations if the predominant diagnosis was severe sepsis, but downgraded the evidence for indirectness.[26] We restricted our settings to the ED, the intensive care unit (ICU) and the operating room. However, since resuscitation from shock should be rare in the elective operative setting, we downgraded the level of evidence in these studies.[28]
Intervention Type(s) (I)
We included studies addressing the use of focused ultrasound or APWA for resuscitative guidance. We excluded studies addressing focused assessment with sonography for trauma or pulse pressure variation (PPV) as we considered these discrete modalities.
Comparison Type(s) (C)
Studies comparing focused ultrasound or APWA to static variables (CVP, pulmonary artery occlusion pressure [PAOP], vital signs) were included in quantitative analysis. We included studies where the comparator was “standard management” but downgraded for bias concerns since protocols were inconsistently defined. We included studies where the comparator was PPV. We excluded comparisons of focused ultrasound to APWA.
Outcome Measure Types (O)
In accordance with GRADE,[13] critical outcomes of mortality, fluid responsiveness and organ failure were selected by the working group. Fluid responsiveness was assessed by CO, stroke volume or any determinant, such as velocity time integrals (VTI). We analyzed organ failures and complications in aggregate since there were a low number of studies that addressed organ failures alone.[29] We then downgraded the evidence for this surrogate outcome.[28]
Methods
Search Strategy
Two searches of PubMed, MEDLINE and the Cochrane Register of Controlled Trials for articles published from January 1, 1992, to December 31, 2016, were performed. The focused ultrasound search included the terms: Bedside Ultrasound, Hemodynamic Ultrasound, focused ultrasound, Point of Care Ultrasound, ICU ultrasound, Limited Ultrasound, Fluid responsiveness, Resuscitation, and Echocardiography. The APWA search included the terms: Arterial waveform analysis, Stroke Volume Variation, Systolic Pressure Variation, noninvasive monitoring, Arterial Pressure Waveform Analysis, Pulse Power Analysis, Pulse Contour Analysis, Transpulmonary Thermodilution, LiDCO, PiCCO, FloTrac, and fluid responsiveness. The “related articles” function and manual review of bibliographies were used to broaden the search.
Study Selection
A team member (D.S.P.) accessed all abstracts and assessed general relevance to our review. A second team member (D.Y.K.) reviewed the determinations. A third team member was available for disagreements. Reviews, case reports, technical papers, letters to the editor, and non-English language publications were excluded. Abstracts were distributed among team members and full text articles were accessed if considered appropriate.
Data Extraction and Management
Data including methodology, population, and outcome, was entered into Review Manager (RevMan) (Version 5.3: Cochrane Collaboration, Oxford). Forest plots were generated when appropriate. The data for fluid responsiveness were used to generate evidence tables.
Assessment of Methodological Quality and Recommendations
Data were entered into GRADEpro (Version 3.2, Cochrane Collaboration, Oxford) to generate quality of evidence tables. However, since the quality of studies evaluating diagnostic test (DTA) accuracy differ; the QUADAS-2 tool[30] was implemented in RevMan to assess methodological quality for fluid responsiveness studies. QUADAS-2 addresses bias and applicability concerns as “low,” “unclear,” or “high” across relevant domains. We also considered the risk-benefit of using the modalities and potential patient and clinician preferences. We prefaced strong recommendations with “we recommend,” and weak recommendations with “we conditionally recommend.”[31]
Data Synthesis and Statistical Analysis
We performed a meta-analysis where adequate data were reported to calculate incidence of our outcomes for comparison. In accordance with recommendations of Cochrane reviews of DTA, pooled sensitivities were not calculated.[32]
Results: Focused Ultrasound
Search Results
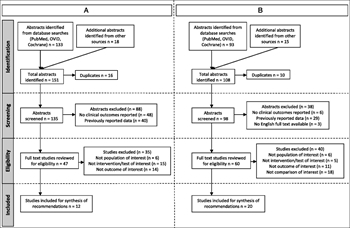
Figure 1. Prisma diagram for systematic review. (A) Prisma diagram of focused ultrasound studies. (B) Prisma diagram of arterial pulse waveform studies.
A total of 151 abstracts were identified (Fig. 1A). After eliminating duplicates, 135 were screened. After exclusions, 47 full text articles were reviewed with 12 studies meeting inclusion criteria.
Results for the Use of Focused Ultrasound for Fluid Responsiveness (PICO 1)
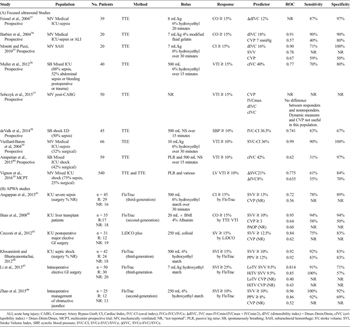
Table 1. Characteristics of Studies Predicting Fluid Responsiveness
Nine studies (73%), reported on fluid responsiveness with most of the population (540 [61%]) from a single multicenter ICU study[10] (Table 1A). Three studies were conducted in medical ICUs in patients with severe sepsis. Only one of these studies and three studies in mixed ICUs reported the percentage of surgical patients (32% and 25%–53%). Two studies relate to surgical subspecialties (Neurosurgery and Cardiothoracic) while the remaining study, with 50% incidence of septic shock, was conducted in an ED. Three studies, one in the ED,[38] and two ICU studies[36][40] addressed nonintubated spontaneously breathing patients. Eight studies measured changes in IVC measurements (by TTE), whereas one study measured SVC (by transesophageal echocardiography) as the index test.[39] Three studies compared focused ultrasound to CVP.
Determinants of fluid responsiveness included increases in CO or VTI. However, one study measured systolic blood pressure response and was downgraded for bias concerns.[38] The reference standards (CO, CI, or VTI) were measured by TTE in six studies, TEE in one study, and by transpulmonary thermodilution[35] in the remaining study. All assigned ideal cutoff points after data collection and analysis, incurring additional bias concerns.
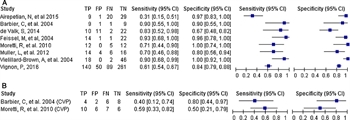
Figure 2. Predictive performance of focused ultrasound versus standard measures to predict fluid responsiveness. (A) Sensitivity and specificity of focused ultrasound to predict fluid responsiveness. (B) Sensitivity and specificity of CVP to predict fluid responsiveness.
One study[37] did not report sensitivities; therefore, only eight studies are included to generate forest plots. Focused ultrasound (Fig. 2A) generally outperformed CVP measures (Fig. 2b). However, four of the nine studies failed to predict fluid responsiveness to predefined tolerance. One of these, in postoperative cardiac patients, showed that focused ultrasound was equivalent to CVP.[37] The three remaining studies that did not demonstrate superiority for focused ultrasound were in spontaneously breathing patients.[36][38][40] In addition, the sensitivities and specificities of the largest study[10] noticeably underperformed compared to smaller previous investigations.
Grading the Evidence PICO Question 1
As per QUADAS 2, risk of bias and applicability concerns were generally “unclear” to “high,” and therefore the overall quality of the evidence as it pertains to PICO 1 is considered low.
Recommendations for the Use of Focused Ultrasound for Fluid Responsiveness (PICO 1)
We conditionally recommend the use of focused ultrasound to determine fluid responsiveness in the management of a mixed population of surgical patients being evaluated for shock. There is a lack of clear superiority of focused ultrasound for this outcome. Focused ultrasound is only useful for the clinician with the training and maintenance of the skill to correctly perform the examination and demonstrates an understanding of the populations of patients that are appropriate for this modality.
Results for the Use of Focused Ultrasound to Reduce Complications and Organ Failures and Complications (PICO 2)
An observational cohort in a mixed ICU of mechanically ventilated patients with undifferentiated shock, assessed organ failures or complications.[37] There was a marked increase in stage III acute kidney injury with standard treatment. Focused ultrasound studies were performed by an American College of Cardiology Level II credentialed intensivist. The protocol was based upon “eyeball” assessments of LV function and IVC fluctuations driving resuscitative decisions.
Grading the Evidence PICO Question 2
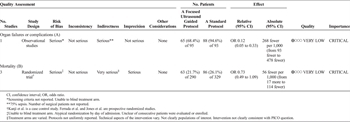
Table 2. Quality Assessment of Studies for Organ Failure or Complications (A) and Mortality (B) Outcomes With Use of Focused Ultrasound
There was serious risk of bias in this one qualifying study for the use of historical controls and for the ill-defined “eye ball” protocol. Additionally, since the number of surgical patients was not reported, there were indirectness concerns. Although the magnitude of effect appears significant, one could not upgrade this single study. The overall quality of the evidence for PICO 2 is very low (Table 2A).
Recommendations for the Use of Focused Ultrasound to Reduce Organ Failures and Complications (PICO 2)
We conditionally recommend the use of focused ultrasound to decrease organ failures and complications in surgical patients being treated for shock. This is based on a lack of high-quality studies that assess organ failures, whereas the single included study had serious methodological concerns. Dependence on focused ultrasound for the purposes of reductions in complications and organ failure should be discouraged outside of an overall protocol.
Results for the Use of Focused Ultrasound to Reduce Mortality (PICO 3)
Mortality was an outcome in three (25%) studies.[21][47][48] Two were prospective randomized trials while the third was that described for PICO 2.[47] Jones et al[48] randomized ED patients in shock to early versus late focused ultrasound and found that the number of potential diagnoses were lower earlier in the treatment group. However, this did not result in a mortality difference. A trauma study[21] assigned patients in shock to LTTE versus no LTTE to assess cardiac function and hypovolemia and guide resuscitation. However, no specific protocols were reported. Mortality trended toward significance but was noteworthy in traumatic brain injury (14.7% vs. 39.5%, p = 0.03). Given the low number of heterogeneous studies, no forest plots were generated for this PICO.
Grading the Evidence PICO Question 3
Risk of bias was serious. Only Jones et al.[48] randomized with a computer-generated sequence while others assigned by day of admission[21] or used historical controls.[47] Only one study[21] relates to the population of interest, and one other study did not address the comparison of interest. Therefore, quality was assessed as very low (Table 2B).
Recommendations for the Use of Focused Ultrasound to Reduce Mortality (PICO 3)
We conditionally recommend the use of focused ultrasound to reduce mortality in surgical patients in shock. This is based on the very low quality of studies related to this outcome. Further, focused ultrasound is only one contributor in an overall protocol designed to improve outcomes; however, protocols were not clearly articulated.
Discussion: Focused Ultrasound
The lack of randomized trials, heterogeneity and indirectness of included studies contributed to our weak recommendations regarding focused ultrasound. A demonstrable cause and effect relationship is lacking. The use of CVP and other static measures (suboptimal resuscitative tools) as the comparator may also artificially skew evidence in favor of focused ultrasound. The risk of faulty interpretation of the focused ultrasound findings can be high[10][24][49] and can lead to medicolegal consequence.[50] Focused ultrasound has a narrow application profile; having been shown to be inaccurate in the setting of arrhythmias, other cardiac dysfunction, early hemorrhage, and spontaneous breathing.[36–38][40][51–53] Despite these limitations, we would presume that some patients and clinicians may prefer a noninvasive means of monitoring. However, an absolute requirement for the use of focused ultrasound is the appropriate training and maintenance of skill needed to perform the examination, interpret the results and understanding of the limitation of the modality.
Application to Clinical Practice
Since this test is operator-dependent, we would recommend enrolling in any of the professional society courses available. Additionally, one could consider ultrasound certification after demonstrating proficiency in logged cases. Thereafter, the clinician would incorporate focused ultrasound into an existing or new protocol and frequently reassess with ongoing QI. The protocol would vary by patient population and clinical circumstance; however, focused ultrasound can be associated with improved performance in the setting of controlled ventilation in the absence of vasopressors or dysrhythmias.
Future Directions
Further study of the use of focused ultrasound in the resuscitation of surgical patients is critical particularly in acute undifferentiated shock and as an adjunct to subsequent resuscitations. Credentialing and certifications in specific technologies is common (eg, mechanical ventilation, fluoroscopy) and will likely apply to focused ultrasound in the future.
Results: Arterial Pulse Waveform Analysis
Search Results
A total of 108 abstracts were identified (Fig. 1B). After eliminating duplicates, 98 were screened. After exclusions, 60 full text articles were reviewed with 20 studies meeting inclusion criteria.
Results for the Use of APWA to Predict Fluid Responsiveness (PICO 4)
Six studies (30%) addressed fluid responsiveness (Table 1B). Three compare SVV with CVP, whereas one compared SVV with CVP and PAOP.[42] The remaining two compared SVV to PPV. Four ICU studies addressed liver transplant patients,[42] postoperative major GI surgery,[43] and septic shock patients.[41][44] The number of surgical patients was not reported in either of these two septic shock studies. Li et al[45] studied the effect of third-generation FloTrac on intraoperative management of major elective GI surgery, whereas Zhao et al.[46] performed an intraoperative analysis of patients with obstructive jaundice. Three studies assessed third-generation FloTrac, two studies evaluated second-generation FloTrac,[42][46] and the remaining referenced the LiDCO device. All patients were mechanically ventilated.
Biais et al.[42] was the only study that used a separate modality (TTE) to measure the reference standard (increases in CO by VTI). The remaining studies utilized FloTrac and LiDCO plus, the study modalities of interest, to measure both the index test and the reference standard introducing unknown confounding. One study compared the APWA device in patients ventilated with traditional versus low tidal volumes showing no difference.[45]
Only one study reported the ideal cutoff for CVP for comparisons.[42] APWA-derived variables generally outperformed non-APWA measures. Forest plots for SVV by APWA and for combined CVP and PPV comparison studies are depicted in Figure 3.
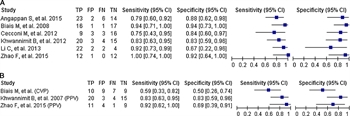
Figure 3. Predictive performance of focused ultrasound versus standard measures to predict fluid responsiveness. (A) Sensitivity and specificity of APWA to predict fluid responsiveness. (B) Sensitivity and specificity of CVP or PPV to predict fluid responsiveness.
Grading the Evidence PICO Question 4
The quality of evidence domains using QUADAS-2[30] was generally “low” to “unclear.” Further, there was high risk of selection bias in two studies where patients were identified on subjective measures for varied indications.[42][44]Applicability concerns were high risk in four (67%) studies. Additionally, unknown confounding is introduced when the same technology is used as the index test and to define the reference standard.
Recommendation for the Use of APWA to Predict Fluid Responsiveness (PICO 4)
We conditionally recommend the use of APWA to predict fluid responsiveness in surgical patients being evaluated for shock. This is based on the concern for applicability and, thus, low quality of the evidence. Similarly, APWA devices should only be used by the clinician who understands its indications and limitations.
Results for the Use of APWA for Reducing Organ Failure and Complications (PICO 5)
Nine (45%) studies referenced organ failures or complications. All were prospective randomized studies except for one retrospective analysis.[54] Seven (78%) investigations were in the intraoperative or early postoperative setting. The remaining were ICU studies. Most studies (55%) analyzed patients undergoing major elective GI surgery while other groups included liver transplant, cardiac surgery, burn and the critically ill patients with severe sepsis. All patients were mechanically ventilated but only three studies reported settings. Two were conventional[55][56] and one utilized “lung protective” modes.[57] Three studies reported organ failures, four reported complications; the remaining reported both. Four reported SOFA scores.
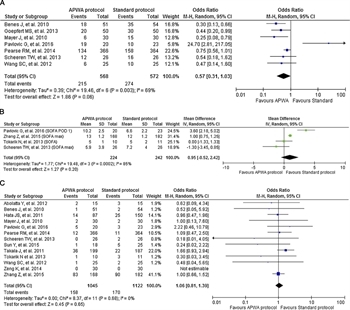
Figure 4. Comparison of APWA versus standard protocols for organ failure, complications and mortality. (A) Comparison of APWA versus standard protocols to reduce organ failures or complications. (B) Comparison of mean difference of SOFA scores associated with APWA versus standard protocols. (C) Comparison of APWA versus standard protocols to reduce mortality.
Results are mixed (Fig. 4). Two major elective GI surgery intraoperative studies (second-generation FloTrac) and one elective cardiac intraoperative study (calibrated PiCCO) favored APWA. The remaining studies showed no significant outcome improvement[54][58][59] with two showing a significant disadvantage with APWA.[60][61] Meta-analysis favored APWA, however high heterogeneity (I[2] = 69%) is demonstrated (Fig. 4a). Further, the four studies that reported SOFA scores trended in favor of standard management (Fig. 4b). Investigations showing no advantage to APWA included “atypical” populations [high-risk emergency surgery,[60] sepsis with ARDS,[61] liver transplant,[54] burn resuscitations,[56]] and/or the use of an uncalibrated device.[58][59]
Grading the Evidence PICO Question 5

Table 3A. Quality Assessment for Complications and Organ Failure Outcomes (a) and Mortality (b) With the Use of APWA Measurements
The quality of the evidence was assessed as “low” for this outcome (Table 3A). Results varied across patient populations and the device used, thus indirectness was a significant concern. Studies were small and confidence intervals were wide.
Recommendation for Use of APWA for Reducing Complications and Organ Failures (PICO 5)
We conditionally recommend the use of APWA to decrease complications or organ failures in surgical patients being treated for shock. This is based on the widely varied results across different populations. Although APWA is favored in select patients yielding meta-analysis results that appear favorable, this should be approached with caution given a low number of high-quality studies. However, patients and clinicians may prefer a less-invasive option with a strong understanding of the limitations.
Results for the Use of APWA Devices to Reduce Mortality (PICO 6)
Thirteen (65%) studies reported mortality outcomes. The majority were prospective randomized trials (77%). Nine (75%) were conducted, at least partially, in the ICU while the remaining were intraoperative studies.[55][59][60][62] Of the six intraoperative studies, five involved major elective GI surgery. Six of the nine ICU studies addressed surgical patients exclusively. The remaining studies were conducted in the setting of acute pancreatitis[63] and severe sepsis.[61][64] All patients were mechanically ventilated. No study showed a significant difference for this outcome with APWA or comparator (Fig. 4C).
Grading the Evidence PICO Question 6

Table 3B. Quality Assessment for Complications and Organ Failure Outcomes (a) and Mortality (b) With the Use of APWA Measurements
Overall grade of the evidence was low (Table 3B). There was concern for indirectness since 50% of studies were conducted intraoperatively or were in the setting of medical critical illness or a low percentage of surgical patients.
Recommendation for the Use of APWA Devices to Decrease Mortality (PICO 6)
We conditionally recommend the use of APWA to reduce mortality. This is based on results that essentially show equivalence to comparators. Any use of APWA mandates a thorough understanding of the narrow clinical application profile supported by published data.
Discussion: APWA
Our recommendations are based on the low quality of the evidence and varied results across populations showing no clear superiority for APWA. The APWA-derived measures may be inaccurate and trend toward inferiority in certain subgroups. Unfortunately, these groups define patients where resuscitative guidance is critical. These include higher acuity abdominal and emergency surgery patients,[58][60][65] severe sepsis,[66–69] burn resuscitations,[56] pressure support ventilation,[68] or any condition where vascular tone is altered due to disease or vasopressors.[54][70–73] APWA is associated with narrow applicability parameters in an acutely unstable mixed population of critically ill.[74] In addition, uncalibrated devices appear to be more prone to error. Further, there is an unknown risk of bias in the many investigations that utilize the same APWA modality to administer the index test as well as to define the reference standard.
Application to Clinical Practice
The technology should be integrated within a resuscitative protocol directed by a clinician who can assimilate the measurements for improved outcomes in the appropriate populations. The new protocol should then undergo ongoing reassessment.
Future Directions
Despite an increasing permeation of these devices, the evidence does not clearly support utilization in surgical patients. Further study is essential particularly in higher acuity patients with ongoing comparison of APWA device types.
Conclusions
Our review reflects the importance of patient selection for focused ultrasound and APWA. Since treatment algorithms were not consistently defined, it was difficult to compare relative performance of focused ultrasound or APWA as diagnostic studies alone or in the context of a treatment protocol. The use of focused ultrasound or APWA requires training and understanding of the measurements as it relates to the specific populations being treated.
A potential weakness of our review is that the acknowledged variability in study types and populations and broad definitions of outcomes make it difficult to address specific knowledge gaps. However, as evidenced by the multitude of focused ultrasound and APWA-based protocols and their use in undifferentiated shock, the review team elected to include the multiple potential roles for these modalities as applied to a broad definition of shock across many populations to identify favorable clinical applications.
Table 4. Summary of Recommendations
Our recommendations are summarized in Table 4. Neither focused ultrasound nor APWA is patently superior to standard protocols in a general population of surgical patients in shock. Therefore, reliance on either or both technologies is not clearly supported for general use. However, with training in identification of appropriate subpopulations, procedural details and interpretation of data, focused ultrasound or APWA can be associated with favorable outcomes when compared with traditional management.
Authorship
V.B. participated in the critical revision. W.C. participated in the taskforce leader, review design, critical revision. S.M.G. participated in the review design, critical revision, writing. M.E.H. participated in the review design, critical revision. U.K. participated in the critical revision. D.Y.K. participated in the review design, data collection, critical revision. D.S.P. is the team leader, and participated in the review design, data collection, writing. A.S.R. participated in the review design, critical revision, writing. B.R.H.R. is the chairperson, and participated in the review design, critical review. S.A.T. participated in the review design, critical revision. J.W. participated in the critical revision. B.W. is the taskforce leader and participated in the critical revision.
Disclosure
The authors declare no conflicts of interest.
References
- Balogh Z, McKinley BA, Cocanour CS, Kozar RA, Holcomb JB, Ware DN, Moore FA. Secondary abdominal compartment syndrome is an elusive early complication of traumatic shock resuscitation. Am J Surg. 2002;184:538–543; discussion 543–4.
- The PRISM Investigators. Early, goal-directed therapy for septic shock - a patient-level meta-analysis. N Engl J Med. 2017;376:2223–2234.
- Angus DC, Barnato AE, Bell D, Bellomo R, Chong CR, Coats TJ, Davies A, Delaney A, Harrison DA, Holdgate A, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med. 2015;41(9):1549–1560.
- PAC-Man study collaboration. Harvey DA, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, Brampton W, Williams D, Young D, Rowan K. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomized controlled trial. Lancet. 2005;366:472–477.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Eng J Med. 2006;354(21):2213–2224.
- Canadian Critical Care Clinical Trials Group. Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, Laporta DP, Viner S, Passerini L, Devitt H, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348(1):5–14.
- Teboul JL, Saugel B, Cecconi M, De Backer D, Hofer CK, Monnet X, Perel A, Pinsky MR, Reuter DA, Rhodes A, et al. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med. 2016;42(9):1350–1359.
- Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(12):1795–1815.
- Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121(6):2000–2008.
- Vignon P, Repessé X, Bégot E, Léger J, Jacob C, Bouferrache K, Slama M, Prat G, Vieillard-Baron A. Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am J Respir Crit Care Med. 2017;195(8):1022–1032.
- FENICE Investigators; ESICM Trial Group. Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, Della Rocca G, Aldecoa C, Artigas A, Jog S, et al. Fluid challenges in intensive care: the FENICE study: a global inception cohort study. Intensive Care Med. 2015;41(9):1529–1537.
- Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, Dahm P. Eastern Association for the Surgery of Trauma Practice Management Guidelines Ad Hoc Committee. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S283–S287.
- Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Available at: http://www.survivingsepsis.org/Guidelines/Pages/default.aspx. Accessed August 26th, 2017.
- International Liaison Committee on Focused Cardiac UltraSound (ILC-FoCUS); International Conference on Focused Cardiac UltraSound (IC-FoCUS). Via G, Hussain A, Wells M, Reardon R, ElBarbary M, Noble VE, Tsung JW, Neskovic AN, Price S, Oren-Grinberg A, et al. International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr. 2014;27(7):683.e1–683.e33.
- Frankel HL, Kirkpatrick AW, Elbarbary M, Blaivas M, Desai H, Evans D, Summerfield DT, Slonim A, Breitkreutz R, Price S, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically Ill Patients—part I: general ultrasonography. Crit Care Med. 2015;43(11):2479–2502.
- Cowie B. Focused cardiovascular ultrasound performed by anesthesiologists in the perioperative period: feasible and alters patient management. J Cardiothorac Vasc Anesth. 2009;23(4):450–456.
- Ha YR, Toh HC. Clinically integrated multi-organ point-of-care ultrasound for undifferentiated respiratory difficulty, chest pain, or shock: a critical analytic review. J Intensive Care. 2016;4:54.
- Jones AE, Craddock PA, Tayal VS, Kline JA. Diagnostic accuracy of left ventricular function for identifying sepsis among emergency department patients with nontraumatic symptomatic undifferentiated hypotension. Shock. 2005;24(6):513–517.
- Melniker LA, Leibner E, McKenney MG, Lopez P, Briggs WM, Mancuso CA. Randomized controlled clinical trial of point-of-care, limited ultrasonography for trauma in the emergency department: the first sonography outcomes assessment program trial. Ann Emerg Med. 2006;48(3):227–235.
- Rozycki GS, Ballard RB, Feliciano DV, Schmidt JA, Pennington SD. Surgeon-performed ultrasound for the assessment of truncal injuries: lessons learned from 1540 patients. Ann Surg. 1998;228(4):557–567.
- Ferrada P, Evans D, Wolfe L, Anand RJ, Vanguri P, Mayglothling J, Whelan J, Malhotra A, Goldberg S, Duane T, et al. Findings of a randomized controlled trial using limited transthoracic echocardiogram (LTTE) as a hemodynamic monitoring tool in the trauma bay. J Trauma Acute Care Surg. 2014;76(1):31–37; discussion 37–8.
- Blanco P, Aguiar FM, Blaivas M. Rapid ultrasound in shock (RUSH) velocity-time integral: a proposal to expand the RUSH protocol. J Ultrasound Med. 2015;34(9):1691–1700.
- Gunst M, Ghaemmaghami V, Sperry J, Robinson M, O'Keeffe T, Friese R, Frankel H. Accuracy of cardiac function and volume status estimates using the bedside echocardiographic assessment in trauma/critical care. J Trauma. 2008;65(3):509–516.
- Strumwasser A, Frankel H, Murthi S, Clark D, Kirton O. Hemodynamic monitoring of the injured patient: from central venous pressure to focused echocardiography. J Trauma Acute Care Surg. 2016;80(3):499–510.
- Levitov A, Frankel HL, Blaivas M, Kirkpatrick AW, Su E, Evans D, Summerfield DT, Slonim A, Breitkreutz R, Price S, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients—part II: cardiac ultrasonography. Crit Care Med. 2016;44(6):1206–1227.
- Carsetti A, Cecconi M, Rhodes A. Fluid bolus therapy: monitoring and predicting fluid responsiveness. Curr Opin Crit Care. 2015;21(5):388–394.
- Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol (1985). 1993;74(5):2566–2573.
- GRADE Working Group; Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Falck-Ytter Y, Jaeschke R, Vist G, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64(12):1303–1310.
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, Alderson P, Glasziou P, Falck-Ytter Y, Schünemann HJ. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64(4):395–400.
- QUADAS-2 Group, Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536.
- Neumann I, Santesso N, Akl EA, Rind DM, Vandvik PO, Alonso-Coello P, Agoritsas T, Mustafa RA, Alexander PE, Schünemann H, et al. A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016;72:45–55.
- Handbook for DTA Reviews. Available at: http://methods.cochrane.org/sdt/handbook-dta-reviews. Accessed December 1, 2016.
- Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30(9):1834–1837.
- Barbier C, Loubières Y, Schmit C, Hayon J, Ricôme JL, Jardin F, Vieillard-Baron A. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30(9):1740–1746.
- Moretti R, Pizzi B. Inferior vena cava distensibility as a predictor of fluid responsiveness in patients with subarachnoid hemorrhage. Neurocrit Care. 2010;13(1):3–9.
- AzuRea group; Muller L, Bobbia X, Toumi M, Louart G, Molinari N, Ragonnet B, Quintard H, Leone M, Zoric L, Lefrant JY. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit Care. 2012;16(5):R188.
- Sobczyk D, Nycz K, Andruszkiewicz P. Bedside ultrasonographic measurement of the inferior vena cava fails to predict fluid responsiveness in the first 6 hours after cardiac surgery: a prospective case series observational study. J Cardiothorac Vasc Anesth. 2015;29(3):663–669.
- de Valk S, Olgers TJ, Holman M, Ismael F, Ligtenberg JJ, Ter Maaten JC. The caval index: an adequate non-invasive ultrasound parameter to predict fluid responsiveness in the emergency department? BMC Anesthesiol. 2014;14:114.
- Vieillard-Baron A, Chergui K, Rabiller A, Peyrouset O, Page B, Beauchet A, Jardin F. Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med. 2004;30(9):1734–1739.
- Airapetian N, Maizel J, Alyamani O, Mahjoub Y, Lorne E, Levrard M, Ammenouche N, Seydi A, Tinturier F, Lobjoie E, et al. Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients? Crit Care. 2015;19:400.
- Angappan S, Parida S, Vasudevan A, Badhe AS. The comparison of stroke volume variation with central venous pressure in predicting fluid responsiveness in septic patients with acute circulatory failure. Indian J Crit Care Med. 2015;19(7):394–400.
- Biais M, Nouette-Gaulain K, Cottenceau V, Revel P, Sztark F. Uncalibrated pulse contour-derived stroke volume variation predicts fluid responsiveness in mechanically ventilated patients undergoing liver transplantation. Br J Anaesth. 2008;101(6):761–768.
- Cecconi M, Monti G, Hamilton MA, Puntis M, Dawson D, Tuccillo ML, Della Rocca G, Grounds RM, Rhodes A. Efficacy of functional hemodynamic parameters in predicting fluid responsiveness with pulse power analysis in surgical patients. Minerva Anestesiol. 2012;78(5):527–533.
- Khwannimit B, Bhurayanontachai R. Prediction of fluid responsiveness in septic shock patients: comparing stroke volume variation by FloTrac/Vigileo and automated pulse pressure variation. Eur J Anaesthesiol. 2012;29(2):64–69.
- Li C, Lin FQ, Fu SK, Chen GQ, Yang XH, Zhu CY, Zhang LJ, Li Q. Stroke volume variation for prediction of fluid responsiveness in patients undergoing gastrointestinal surgery. Int J Med Sci. 2013;10(2):148–155.
- Zhao F, Wang P, Pei S, Mi W, Fu Q. Automated stroke volume and pulse pressure variations predict fluid responsiveness in mechanically ventilated patients with obstructive jaundice. Int J Clin Exp Med. 2015;8(11):20751–20759. eCollection 2015.
- Kanji HD, McCallum J, Sirounis D, MacRedmond R, Moss R, Boyd JH. Limited echocardiography-guided therapy in subacute shock is associated with change in management and improved outcomes. J Crit Care. 2014;29(5):700–705.
- Jones AE, Tayal VS, Sullivan DM, Kline JA. Randomized, controlled trial of immediate versus delayed goal-directed ultrasound to identify the cause of nontraumatic hypotension in emergency department patients. Crit Care Med. 2004;32(8):1703–1708.
- Expert Round Table on Echocardiography in ICU. Vieillard-Baron A, Mayo PH, Vignon P, Cholley B, Slama M, Pinsky MR, McLean A, Choi G, Beaulieu Y, Arntfield R, et al. International consensus statement on training standards for advanced critical care echocardiography. Intensive Care Med. 2014;40(5):654–666.
- Mayo P, Mekontso Dessap A, Vieillard-Baron A. Myths about critical care echocardiography: the ten false beliefs that intensivists should understand. Intensive Care Med. 2015;41(6):1103–1106.
- Boyd JH, Sirounis D, Maizel J, Slama M. Echocardiography as a guide for fluid management. Crit Care. 2016;20:274.
- Juhl-Olsen P, Vistisen ST, Christiansen LK, Rasmussen LA, Frederiksen CA, Sloth E. Ultrasound of the inferior vena cava does not predict hemodynamic response to early hemorrhage. J Emerg Med. 2013;45(4):592–597.
- Corl K, Napoli AM, Gardiner F. Bedside sonographic measurement of the inferior vena cava caval index is a poor predictor of fluid responsiveness in emergency department patients. Emerg Med Australas. 2012;24(5):534–539.
- Wang SC, Teng WN, Chang KY, Susan Mandell M, Ting CK, Chu YC, Loong CC, Chan KH, Tsou MY. Fluid management guided by stroke volume variation failed to decrease the incidence of acute kidney injury, 30-day mortality, and 1-year survival in living donor liver transplant recipients. J Chin Med Assoc. 2012;75(12):654–659.
- Benes J, Chytra I, Altmann P, Hluchy M, Kasal E, Svitak R, Pradl R, Stepan M. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care. 2010;14(3):R118.
- Goepfert MS, Richter HP, Zu Eulenburg C, Gruetzmacher J, Rafflenbeul E, Roeher K, von Sandersleben A, Diedrichs S, Reichenspurner H, Goetz AE, et al. Individually optimized hemodynamic therapy reduces complications and length of stay in the intensive care unit: a prospective, randomized controlled trial. Anesthesiology. 2013;119(4):824–836.
- okarik M, Sjöberg F, Balik M, Pafcuga I, Broz L. Fluid therapy LiDCO controlled trial-optimization of volume resuscitation of extensively burned patients through noninvasive continuous real-time hemodynamic monitoring LiDCO. J Burn Care Res. 2013;34(5):537–542.
- OPTIMISE Study Group; Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, Grocott MP, Ahern A, Griggs K, Scott R, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311(21):2181–2190.
- Scheeren TW, Wiesenack C, Gerlach H, Marx G. Goal-directed intraoperative fluid therapy guided by stroke volume and its variation in high-risk surgical patients: a prospective randomized multicentre study. J Clin Monit Comput. 2013;27(3):225–233.
- Pavlovic G, Diaper J, Ellenberger C, Frei A, Bendjelid K, Bonhomme F, Licker M. Impact of early haemodynamic goal-directed therapy in patients undergoing emergency surgery: an open prospective, randomised trial. J Clin Monit Comput. 2016;30(1):87–99.
- Zhang Z, Ni H, Qian Z. Effectiveness of treatment based on PiCCO parameters in critically ill patients with septic shock and/or acute respiratory distress syndrome: a randomized controlled trial. Intensive Care Med. 2015;41(3):444–451.
- Mayer J, Boldt J, Mengistu AM, Röhm KD, Suttner S. Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: a randomized, controlled trial. Crit Care. 2010;14(1):R18.
- Sun Y, Lu ZH, Zhang XS, Geng XP, Cao LJ, Yin L. The effects of fluid resuscitation according to PiCCO on the early stage of severe acute pancreatitis. Pancreatology. 2015;15(5):497–502.
- Takala J, Ruokonen E, Tenhunen JJ, Parviainen I, Jakob SM. Early non-invasive cardiac output monitoring in hemodynamically unstable intensive care patients: a multi-center randomized controlled trial. Crit Care. 2011;15(3):R148.
- Lahner D, Kabon B, Marschalek C, Chiari A, Pestel G, Kaider A, Fleischmann E, Hetz H. Evaluation of stroke volume variation obtained by arterial pulse contour analysis to predict fluid responsiveness intraoperatively. Br J Anaesth. 2009;103(3):346–351.
- Ganter MT, Alhashemi JA, Al-Shabasy AM, Schmid UM, Schott P, Shalabi SA, Badri AM, Hartnack S, Hofer CK. Continuous cardiac output measurement by un-calibrated pulse wave analysis and pulmonary artery catheter in patients with septic shock. J Clin Monit Comput. 2016;30(1):13–22.
- Machare-Delgado E, Decaro M, Marik PE. Inferior vena cava variation compared to pulse contour analysis as predictors of fluid responsiveness: a prospective cohort study. J Intensive Care Med. 2011;26(2):116–124.
- Perner A, Faber T. Stroke volume variation does not predict fluid responsiveness in patients with septic shock on pressure support ventilation. Acta Anaesthesiol Scand. 2006;50(9):1068–1073.
- Slagt C, Malagon I, Groeneveld AB. Systematic review of uncalibrated arterial pressure waveform analysis to determine cardiac output and stroke volume variation. Br J Anaesth. 2014;112(4):626–637.
- Suehiro K, Tanaka K, Matsuura T, Funao T, Yamada T, Mori T, Nishikawa K. The Vigileo-FloTrac™ system: arterial waveform analysis for measuring cardiac output and predicting fluid responsiveness: a clinical review. J Cardiothorac Vasc Anesth. 2014;28(5):1361–1374.
- Metzelder S, Coburn M, Fries M, Reinges M, Reich S, Rossaint R, Marx G, Rex S. Performance of cardiac output measurement derived from arterial pressure waveform analysis in patients requiring high-dose vasopressor therapy. Br J Anaesth. 2011;106(6):776–784.
- Monnet X, Anguel N, Jozwiak M, Richard C, Teboul JL. Third-generation FloTrac/Vigileo does not reliably track changes in cardiac output induced by norepinephrine in critically ill patients. Br J Anaesth. 2012;108(4):615–622.
- Meng L, Tran NP, Alexander BS, Laning K, Chen G, Kain ZN, Cannesson M. The impact of phenylephrine, ephedrine, and increased preload on third-generation Vigileo-FloTrac and esophageal Doppler cardiac output measurements. Anesth Analg. 2011;113(4):751–757.
- Delannoy B, Wallet F, Maucort-Boulch D, Page M, Kaaki M, Schoeffler M, Alexander B, Desebbe O. Applicability of pulse pressure variation during unstable hemodynamic events in the intensive care unit: a five-day prospective multicenter study. Crit Care Res Pract. 2016;2016:7162190.