Nutritional Support in Trauma Patients (UPDATE IN PROCESS)
Published 2004
Citation: J Trauma. 57(3):660-679, September 2004.
Authors
Jacobs, David G. MD; Jacobs, Danny O. MD; Kudsk, Kenneth A. MD; Moore, Frederick A. MD; Oswanski, Michael F. MD; Poole, Galen V. MD; Sacks, Gordon S. PharmD; Scherer, LR Tres III MD; Sinclair, Karlene E. MD; the EAST Practice Management Guidelines Work Group
Author Information
From the Department of Surgery, Carolinas Medical Center (D.G.J.), Charlotte, North Carolina, Department of Surgery, Creighton University School of Medicine (D.O.J.), Omaha, Nebraska, Department of Surgery, University of Tennessee (K.A.K.), Memphis, Memphis, Tennessee, Department of Surgery, University of Texas Medical School (F.A.M.), Houston, Texas, Trauma Services, The Toledo Hospital (M.F.O.), Toledo, Ohio, Department of Surgery (G.V.P.) and School of Pharmacy (G.S.S.), University of Mississippi Medical Center, Jackson, Mississippi, Division of Pediatric Surgery, Indiana University School of Medicine (L.R.S.), Indianapolis, Indiana, and Department of Surgery, Morehouse School of Medicine (K.E.S.), Atlanta, Georgia.
Address for reprints: David G. Jacobs, MD, Department of Surgery, Carolinas Medical Center, P.O. Box 32861, Charlotte, NC 28232-2861; email: david.jacobs@carolinashealthcare.org.
Introduction
Nutritional support is an integral, though often neglected, component of the care of the critically injured patient. Our understanding of the metabolic changes associated with starvation, stress, and sepsis has deepened over the past 20 to 30 years, and along with this has come a greater appreciation for the importance of the timing, composition, and route of administration of nutritional support to the trauma patient. While supportive data exist for many of our current nutritional practices, the trauma surgeon cannot assume that interventions which are successful in laboratory animals or even in the critically-ill non-trauma patient will produce the same results in critically ill trauma patients. Stanley J. Dudrick, MD, one of the forefathers of surgical nutrition in this country, put it this way. “…we do get ourselves into an awful lot of trouble and lack of consensus as a result of mixing in animal data together with normal, starved man data when we are talking about trauma, especially in burns.”[1] For this reason, the recommendations provided in this guideline are based, when at all possible, on studies using trauma or burn patients. Nevertheless, a brief discussion of some of the basic science principles of nutritional support is provided in the following section as a backdrop for the clinical studies presented in this guideline.
Individual Guidelines
This practice management guideline is a compilation of six separate guidelines; each addresses a specific aspect of the nutritional support of the trauma patient. These topics are presented in the following order:
A. Route of Nutritional Support (Total Parenteral Nutrition versus Total Enteral Nutrition)
B. Timing of Nutritional Support (Early versus Late)
C. Site of Nutritional Support (Gastric versus Jejunal)
E. Monitoring of Nutritional Support (Which tests and how often?)
F. Type of Nutritional Support (Standard versus Enhanced)
Each sub-guideline is a separate and free-standing document, with its own recommendations, evidentiary tables, and references. Where possible, we have attempted to eliminate redundancy, and ensure consistency amongst the guidelines. Yet, due to substantial differences in both the quantity as well as the quality of supporting scientific data for each topic, and the fact that certain clinical circumstances are not conducive to a single guideline, concise and consistent recommendations were not always possible. Even when Class I (prospective randomized controlled) studies were available, limited patient numbers, and inconsistent definitions rendered study conclusions less authoritative that they might have otherwise been. Recognizing the need to incorporate the major recommendations from the sub-guidelines into a logical overall approach to the nutritional support of the trauma patient, a summary algorithm is provided at the conclusion of the guideline (Figure 1.). Due to the scope of this document, many of the recommendations from the sub-guidelines could not be included in the algorithm. In addition, distinguishing between the various levels of recommendations (I, II, and III) within the algorithm was not practical. Nevertheless, the algorithm provides a safe, reasonable, and literature-supported approach to nutritional support and, we hope, will provoke constructive discussion and stimulate further investigation.
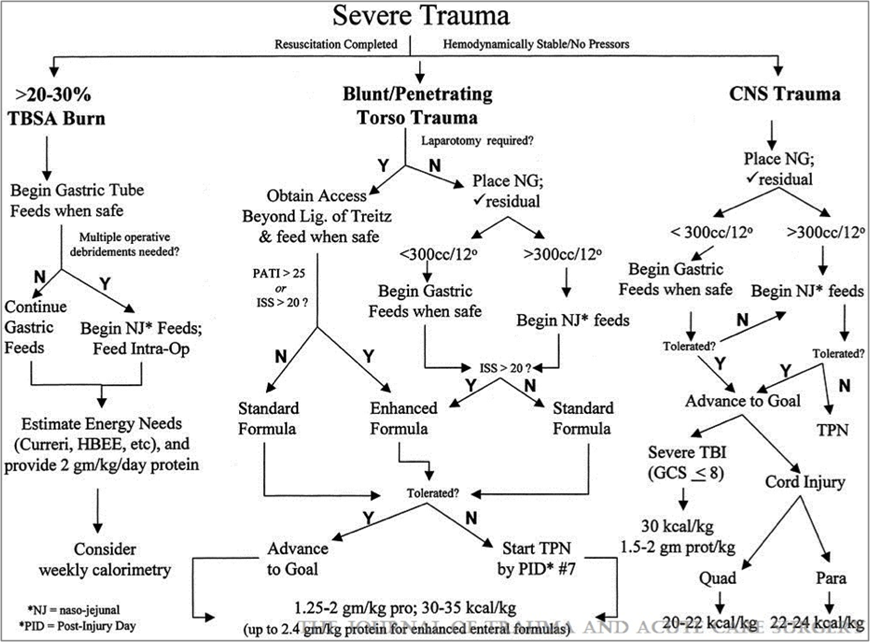
Fig. 1: Summary algorithm for nutritional support of the trauma patient.
Experimental Background
The first suggestion that route and type of nutrition influence clinical outcome was made in a study by Alexander and others, which included severely burned patients randomized to a standard enteral diet or a protein-supplemented diet.[2] Children receiving the high-protein enteral diet had a higher survival rate and fewer septic complications than children receiving the standard enteral diet. Although not discussed at the time, patients receiving the high-protein diet were administered significantly less parenteral nutrition than the standard diet group. During the same time period, experimental observations depicted differences between enteral and parenteral feeding. In a model of septic peritonitis, both malnourished and well-nourished animals administered the total parenteral nutrition (TPN) solution enterally survived peritonitis significantly better than animals pair fed the TPN solution intravenously.[3] [4] Since these initial studies, many clinical trials have studied the impact of route and type of nutrition comparing enterally fed patients (receiving a variety of enteral products) with 1) unfed trauma patients[5] [6] and 2) trauma[7-9] or burn patients[10] given IV-TPN. In addition, burn patients have been studied after receiving a variety of enteral formulas (high versus standard protein,[2] enhanced versus standard diet[11]), while patients sustaining severe head injury have been randomized to intravenous nutrition versus intragastric feeding[12] [13] or intragastric versus postpyloric feeding.[14] [15]
While the preponderance of these studies show benefits of the enteral route with additional improvement with various specialty substrates in select patient populations, investigators have searched for mechanisms to explain improved infectious rates with enteral feeding. Intravenous feeding increases gut permeability[16] [17] and increases bacterial translocation to mesenteric lymph nodes,[18] [19] connoting a breakdown in the gut mucosal barrier that allows passage of small and large molecules from the intestinal lumen. Experimentally, bacterial translocation increases with intravenous nutrition, an enteral elemental diet, burns, hemorrhage, and shock, but not with starvation alone unless a simultaneous inflammatory focus is created.[20] Inflammatory molecules, such as zymosan, also increase gut permeability to bacteria.[21] Reduction in IgA and increases in bacterial translocation occur with bacterial overgrowth within the gastrointestinal tract (primarily aerobic bacteria).[19] These permeability increases to macromolecules have been noted in burn patients[22] [23] and patients sustaining blunt and penetrating trauma to the torso.[24] [25] Numerous investigations into the significance of bacterial translocation have engendered a hypothesis that the permeable gut allows systemic entry of toxic substances with deleterious end organ effects, but this work has not shown a relationship between increased permeability and the development of intra-abdominal or pulmonary infectious complications. Recently, the gastrointestinal tract has been defined as a site for leukocyte “priming” following initial injury which up-regulates the inflammatory response in the lungs after a secondary hit.[26-28] Manipulation of this initial priming via the gastrointestinal tract is a current focus of investigation.
Investigations into the nutrient manipulation of the mucosal immune system also provide an intriguing insight into the host defenses at mucosal surfaces. Mucosal associated lymphoid tissue which originated from gut-associated lymphoid tissue (GALT) accounts for approximately 50% of the total body’s immunity and 70%-80% of immunoglobulin production by the body, primarily in the form of IgA.[29] Experimentally, dietary conditions which increase bacterial translocation (IV-TPN or an elemental diet) are associated with significant reductions in GALT cells within the Peyer’s patches, lamina propria, and intraepithelial space in association with decreases in intestinal and respiratory IgA levels.[30] Functionally, the hypoplasia of this GALT system induced by inadequate nutrient regimens impair IgA-mediated antiviral mucosal immunity[31] and resistance to established immunity against intratracheal Pseudomonas.[32] This deterioration may be associated with loss of systemic immunity with impaired function of polymorphonuclear cells and monocytes. Experimentally, reduction in IgA levels in vitro increase the virulence of intraluminal bacteria improving bacterial ability to attach, and potentially invade, mucosal surfaces.[33] These experimental manipulations serve as a backdrop for our understanding of the clinical studies of route and type of nutrition in patients sustaining severe trauma, burns, or head injury.
References
- Dudrick SJ. Nutritional therapy in burned patients. J Trauma. 1979;19:908-909.
- Alexander JW, Macmillan BG, Stinnett JD, et al. Beneficial effects of aggressive protein feeding in severely burned children. Ann Surg. 1980;192:505-517.
- Kudsk KA, Carpenter G, Petersen SR, Sheldon GF. Effective enteral and parenteral feeding in malnourished rats with E. coli-hemoglobin adjuvant peritonitis. J Surg Res. 1981;31:105110.
- Kudsk KA, Stone JM, Carpenter G, Sheldon GF. Enteral and parenteral feeding influences mortality after hemoglobin-E. coli peritonitis in normal rats. J Trauma. 1983;23:605-609.
- Moore FA, Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma - A prospective randomized study. J Trauma. 1986;26:874-881.
- Kudsk KA, Minard G, Croce MA, et al. A randomized trial of isonitrogenous enteral diets following severe trauma. An immune-enhancing diet reduces septic complications. Ann Surg. 1996;224:531-543.
- Moore FA, Moore EE, Jones TN, McCroskey BL, Peterson VM. TEN versus TPN following major abdominal trauma - reduced septic morbidity. J Trauma. 1989 ;29:916-923.
- Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding: effects on septic morbidity following blunt and penetrating trauma. Ann Surg. 1992;215:503-513.
- Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications: the results of a meta-analysis. Ann Surg. 1992;216:172-183.
- Herndon DN, Barrow RE, Stein M, et al. Increased mortality with intravenous supplemental feeding in severely burned patients. J Burn Care Rehabil. 1989;10:309-313.
- Gottschlich MM, Jenkins M, Warden GD, et al. Differential effects of three enteral dietary regimens on selected outcome variables in burn patients. J Parenter Enteral Nutr. 1990;14:225-236.
- Rapp RP, Young B, Twyman D, et al. The favorable effect of early parenteral feeding on survival in head-injured patients. J Neurosurg. 1983;58:906-912.
- Young B, Ott L, Twyman D, et al. The effect of nutritional support on outcome from severe head injury. J Neurosurg. 1987;67:668-676.
- Grahm TW, Zadrozny DB, Herrington T. The benefits of early jejunal hyperalimentation in the head-injured patient. Neurosurgery. 1989;25:729-735.
- Borzotta AP, Penning J, Papasadero B, et al. Enteral versus parenteral nutrition after severe closed head injury. J Trauma. 1994; 37:459-468.
- Rothman D, Latham MC, Walker WA. Transport of macromolecules in malnourished animals: Evidence of increased uptake of intestinal antigens. Nutr Res. 1982;2:467.
- Purandare S, Offenbartl K, Westrom B, Bengmark S. Increased gut permeability to fluorescein isothiocyanate-dextran after total parenteral nutrition in rat. Scand J Gastroenterol. 1989;24:678-682.
- Deitch EA, Winterton J, Li M, Berg R. The gut as a portal of entry for bacteremia. Role of protein malnutrition. Ann Surg. 1987;205:681-692.
- Alverdy JC, Aoys E, Moss GS. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery. 1988;104:185-190.
- Deitch EA. Does the gut protect or injure patients in the ICU? Perspect Crit Care. 1988;1:1-31.
- Deitch EA, Specian RD, Grisham MB, Berg RD. Zymosan-induced bacterial translocation: a study of mechanisms. Crit Care Med. 1992;20:782-788.
- Deitch EA. Intestinal permeability is increased in burn patients shortly after injury. Surgery. 1990;107:411-416.
- Ziegler TR, Smith RJ, O'Dwyer ST, Demling RH, Wilmore DW. Increased intestinal permeability associated with infection in burn patients. Arch Surg. 1988;123:1313-1319.
- Langkamp-Henken B, Donovan TB, Pate LM, Maull CD, Kudsk KA. Increased intestinal permeability following blunt and penetrating trauma. Crit Care Med. 1995;23:660-664.
- Janu P, Li J, Minard G, Kudsk K. Systemic interleukin-6 (IL-6) correlates with intestinal permeability. Surg Forum. 1996;47:7-9.
- Koike K, Moore EE, Moore FA, Franciose RJ, Fontes B, Kim FJ. CD11b blockade prevents lung injury despite neutrophil priming after gut ischemia/reperfusion. J Trauma. 1995;39:23-27.
- Kim FJ, Moore EE, Moore FA, Biffl WL, Fontes B, Banerjee A. Reperfused gut elaborates PAF that chemoattracts and primes neutrophils. J Surg Res. 1995;58:636-640.
- Moore EE, Moore FA, Franciose RJ, Kim FJ, Billf WL, Banerjee A. The post-ischemic gut serves as a priming bed for circulating neutrophils that provoke multiple organ failure. J Trauma. 1994;37:881-887.
- Langkamp-Henken B, Glezer JA, Kudsk KA. Immunologic structure and function of the gastrointestinal tract. Nutr Clin Pract. 1992;7:100-108.
- Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langkamp-Henken B. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39:44-52.
- Kudsk KA, Li J, Renegar KB. Loss of upper respiratory tract immunity with parenteral feeding. Ann Surg. 1996;223:629-638.
- King BK, Kudsk KA, Li J, Wu Y, Renegar KB. Route and type of nutrition influence mucosal immunity to bacterial pneumonia. Ann Surg. (In Press)
- Svanborg C. Bacterial adherence and mucosal immunity. In: Ogra PL, Lamm ME, McGhee JR, Mestecky J, Strober W, Bienenstock J, eds. Handbook of Mucosal Immunology. San Diego, CA: Academic Press, Inc.; 1974:71-78.