Open Abdomen in Trauma and Emergency General Surgery, Management of: Part 1
Published 2010
Citation: J Trauma. 68(6):1425-1438, June 2010
Authors
Diaz, Jose J. Jr. MD; Cullinane, Daniel C. MD; Dutton, William D. MD; Jerome, Rebecca MS; Bagdonas, Richard MD; Bilaniuk, Jarolslaw O. MD; Collier, Bryan R. DO; Como, John J. MD; Cumming, John MD; Griffen, Maggie MD; Gunter, Oliver L. MD; Kirby, John MD; Lottenburg, Larry MD; Mowery, Nathan MD; Riordan, William P. Jr. MD; Martin, Niels MD; Platz, Jon MD; Stassen, Nicole MD; Winston, Eleanor S. MD
Author Information
From the Division of Trauma and Surgical Critical Care (J.J.D., B.R.C., W.D.D., R.N.J., O.G., W.R.), Department of Surgery, Vanderbilt University Medical Center, Nashville, Tennesse; Division of Trauma, Critical Care and General Surgery (D.C.C.), Mayo Clinic, Rochester, Minnesota; Division of Trauma Surgery and Division of Surgical Critical Care (R.B.), Nassau University Medical Center, East Meadow, New York; Department of Emergency Medicine (J.W.B.), Morristown Memorial Hospital,, Morristown, New Jersey; Division of Trrauma, Critical Care, Burns, and Metro Life Flight (J.J.C.), MetroHeath Medical Center, Cleveland, Ohio; Department of Surgery (J.K.C.), North Trauma Services, Orono, Minnesota; Inova Regional Trauma Center (M.M.G.), Inova Fairfax Hospital, Fairfax, Virginia; Division of Burn, Trauma, Critical Care Surgery (J.P.K.), Washington University Medical Center, St. Louis, Missouri; Division of Acute Care Surgery (L.L.), University of Florida College of Medicine, Gainesville, Florida; Division of Traumatology, Surgical Critical Care and Emergency Surgery (N.M.), University of Pennsylvania, Philadelphia, Pennsylvania; Department of Surgery (N.T.M.), Wake Forest University School of Medicine, Winston-Salem, North Carolina; Division of Surgical Critical Care/Trauma (J.J.P.), North Shore University Hospital Long Island Jewish Medical Center, Manhasset, New York; Acute Care and Trauma Surgery Division, Strong Regional Trauma Center (N.A.S.), University of Rochester Medical Center, Rochester, New York; and Division of Trauma and Emergency Surgery Services (E.S.W.), Baystate Surgical Associates, Springfield, Massachusetts.
Submitted for publication June 2, 2009.
Accepted for publication January 15, 2010.
Presented at the 22nd Annual Meeting of the Eastern Association for the Surgery of Trauma, January 13–17, 2009, Lake Buena Vista, Florida.
Address for reprints: Jose J. Diaz, Jr., MD, CNS, FACS, FCCM, Division of Trauma and Surgical Critical Care, Department of Surgery, Vanderbilt University Medical Center, 1211 21st Avenue South, 404 Medical Arts Building, Nashville, TN 37212; email: jose.diaz@vanderbilt.edu.
Abstract
Background: The open abdomen technique, after both military and civilian trauma, emergency general or vascular surgery, has been used in some form for the past 30 years. There have been several hundred citations on the indications and the management of the open abdomen. Eastern Association for the Surgery of Trauma practice management committee convened a study group to organize the world's literature for the management of the open abdomen. This effort was divided into two parts: damage control and the management of the open abdomen. Only damage control is presented in this study. Part 1 is divided into indications for the open abdomen, temporary abdominal closure, staged abdominal repair, and nutrition support of the open abdomen.
Methods: A literature review was performed for more than 30 years. Prospective and retrospective studies were included. The reviews and case reports were excluded. Of 1,200 articles, 95 were selected. Seventeen surgeons reviewed the articles with four defined criteria. The Eastern Association for the Surgery of Trauma primer was used to grade the evidence.
Results: There was only one level I recommendation. A patient with documented abdominal compartment syndrome should undergo decompressive laparotomy.
Conclusion: The open abdomen technique remains a heroic maneuver in the care of the critically ill trauma or surgical patient. For the best outcomes, a protocol for the indications, temporary abdominal closure, staged abdominal reconstruction, and nutrition support should be in place.
The management of catastrophic abdominal injuries has been described in past military conflicts. One of the initial references of the use of open abdomen technique was by Ogilvie[1] in 1940 during World War II.
“A dodge that has twice helped me in a difficulty is the use of light canvas or stout cotton cloth sterilized in Vaseline. A double sheet of this is cut rather smaller than the defect in the muscles, and sutured into place with interrupted catgut sutures . . .. This device is obviously temporary, but it prevents retraction of the edges of the gap, it keeps the intestinal contents from protruding during the early days when they are so difficult to retain, and it allows the abdominal wall to be used as a whole in respiration.”
Subsequently, the open abdomen technique has gone through various evolutions, and many surgeons since have refined the technique. Stone and Lamb,[2] Stone et al.,[3] Lucas and Ledgerwood,[4] and Rotondo et al.[5]helped usher in the modern era of “damage control” (DC) in trauma surgery. In addition, the use of the open abdomen (OA) technique has been used in the management of emergency general surgery, vascular surgery, intra-abdominal sepsis, and acute pancreatitis. Abdominal compartment syndrome (ACS) after ruptured abdominal aortic aneurysm (rAAA) or trauma has become one of the key life-saving indications for decompressive laparotomy and open abdomen technique.
During the course of the past 30 years, several authors have contributed their clinical experience to the literature in an effort to define the clinical indications and to describe the various management strategies for the appropriate use of the open abdomen technique. There has remained a great degree of heterogeneity in the patient populations, and the surgical techniques described. The OA approach is used in both military and civilian trauma, vascular emergencies, and emergency general surgery. Given the lack of consensus, the Eastern Association for the Surgery of Trauma Practice Management Guidelines Committee convened a study group to establish the recommendations for the use of OA techniques in both trauma and nontrauma surgery and to provide guidelines regarding the following specific topics:
- Indications for OA technique in ACS, DC, general surgery, and vascular surgery.
- Surgical technique for temporary abdominal closure (TAC).
- Surgical technique for repeat laparotomy and staged abdominal reconstruction (STAR).
- Nutritional aspects of open abdomen technique.
Process
A computerized search of the National Library of Medicine Medline database was undertaken using the PubMed Entrez interface. The citations in English were identified during the period of 1984 through 2009 using the primary search strategies outlined. Given the complexity of this literature, several strategies were necessary to appropriately capture the breadth of evidence on the topic. The search excluded case reports, reviews, letters or commentary, editorials, and articles focusing only on pediatric participants.
The PubMed-related articles algorithm was also used to identify the additional articles similar to the items retrieved by the primary strategy, in addition to hand searching of the reference lists of key articles retrieved by the searches. Of approximately 1,200 articles identified by these two techniques, only prospective or retrospective studies examining open abdominal management were selected, consisting of 145 institutional studies evaluating open abdomen management strategies in the adult surgical or critical care population. Ninety-five articles pertained to the topics studied and were used to develop the recommendations. The articles were reviewed by a group of 18 surgeons who collaborated to produce this practice management guideline. The chair, vice chair, and a committee member (J.J.D., D.C., and W.D.) reviewed all the articles to categorize them into the four study topics. They were then distributed to all members of the study group for critical review. Each committee member has to answer the following four questions of each article reviewed:
- What is the class of evidence in the article?
- Are the results of the article valid based on the data presented?
- What is your conclusion based on the evidence the article provided?
- Does the article supports the class of evidence?; can it be evaluated using Eastern Association for the Surgery of Trauma Primer guidelines?
The correlation between the evidence and the level of recommendations is as follows:
Level I
This recommendation is convincingly justifiable based on the available scientific information alone. It is usually based on class I data; however, strong class II evidence may form the basis for a level I recommendation, especially if the issue does not lend itself to testing in a randomized format. Conversely, weak or contradictory class I data may not be able to support a level I recommendation.
Level II
This recommendation is reasonably justifiable by available scientific evidence and strongly supported by expert opinion. It is usually supported by class II data or a preponderance of class III evidence.
Level III
This recommendation is supported by available data but adequate scientific evidence is lacking. It is generally supported by class III data. This type of recommendation is useful for educational purposes and in guiding future studies.
Recommendations
Indications for the Use of the Open Abdomen Technique
Abdominal Compartment Syndrome
-
All patients with ACS, defined as intra-abdominal pressure (IAP) >20 mm Hg (with or without an abdominal perfusion pressure (APP) ≤60 mm Hg—World Congress of ACS [WCASC] definition), manifested as organ dysfunction (abdominal distension, decompensating cardiac, pulmonary, and renal dysfunction) should undergo emergent or urgent decompressive laparotomy (Table 1) (level I).
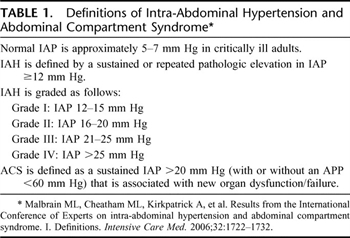
Table 1. Definitions of Intra-Abdominal Hypertension and Abdominal Compartment Syndrome
- An acute increase of intra-abdominal pressures to ≥25 mm Hg, ACS is likely and decompressive laparotomy and an open abdomen technique should be considered (level II).
-
After DC of nonabdominopelvic trauma, IAP should be monitored as secondary ACS can occur after either massive transfusion or massive fluid resuscitation (Table 2; Fig. 1, see flow diagram) (level II).

Table 2. Abdominal Compartment Syndrome Types
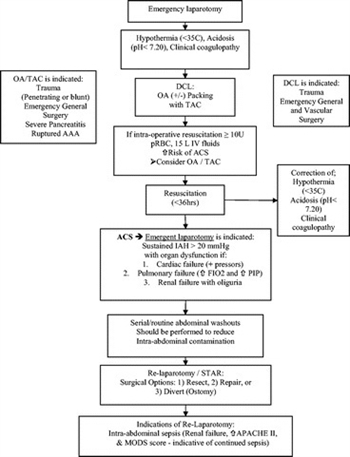
Figure 1. The management of the open abdomen in trauma, emergency general, and vascular surgery flow diagram. OA, open abdomen; PIP, peak inspiratory pressure.
- After DC with an open abdomen, IAP should be monitored, as continued massive resuscitation can cause recurrent ACS (Table 2) (level II).
- Open abdomen management should be considered in the following clinical circumstance as prevention of ACS: transfusion >10 units of red blood cell (RBC) and fluid resuscitation >15 L of crystalloid (level III).
- With Intraabdominal hypertension (IAH) >20 mm Hg (grade III WCACS definitions), one should monitor for potential organ dysfunction, and if present, should consider the following (Tables 1 and 2): (1) increase sedation/neuromuscular blockade/body positioning (level II), (2) evacuate intra-abdominal fluid collections (level III), (3) correct positive fluid balances: colloid versus crystalloid, diuresis, fluid restrictions (level II), (4) abdominal decompression (level II).
Damage Control
- There are no level I recommendations for DC in trauma, emergency general surgery, or vascular emergencies.
- In the cases of severe abdominal trauma because of penetrating or blunt injury involving hepatic, nonhepatic, or vascular injuries with intra-abdominal packing, the use of the OA technique should be considered, and an early decision to truncate a definitive operation should be made as soon as possible (level II).
- DC and the OA technique should be considered if the following clinical parameters are reached: acidosis (pH ≤7.2), hypothermia (temperature ≤35°C), and clinical coagulopathy and or if the patient is receiving massive transfusion (≥10 units packed RBCs [PRBCs]) (level III).
Emergency General Surgery
- The DC and open abdomen technique may be considered in patients with severe intra-abdominal infection/peritonitis. Source control remains the major predictor of outcome (level II).
- The DC and open abdomen technique may be considered in the management of severe necrotizing pancreatitis (level III).
Vascular Surgery
The DC and open abdomen technique should be considered after rAAA in the following clinical circumstances:
- Significant visceral edema where abdominal closure would result in ACS (level II).
- IAH of 21 mm Hg in postoperative rAAA (level III).
Temporary Abdominal Closure
- There are no level I recommendations for TAC in trauma, emergency general surgery, or vascular emergencies.
- Any TAC technique must provide for easy re-exploration, a high rate of definitive closure, and be cost effective (level II).
- Multiple techniques of TAC are safe including Bogotá bag, Wittman Patch, and Vacuum pack (VP). All allow ready access for relaparotomy procedures and provide tension-free closure contributing to the prevention of IAH (level II).
- Permanent mesh (i.e., polypropylene [PPE]) should not be used for TAC, as it is associated with high fistula rates (level III).
- The 3-layer VP (protective barrier against the viscera, surgical towel, drains, and occlusive adhesive drape) is considered the current standard by which to measure other devices (level III).
Relaparotomy/Staged Abdominal Reconstruction
- There are no level I recommendations for relaparotomy, on-demand laprotomy or STAR in trauma, emergency general surgery, or vascular emergencies.
- Primary closure of the abdominal wall should be performed when possible.
- On-demand laparotomy is associated with a reduction in relaparotomies and negative laparotomies that may reduce healthcare utilization and medical costs (level II).
- Planned and on-demand relaparotomy can be considered in both abdominal sepsis and necrotizing pancreatitis. When primary closures can be achieved, on-demand relaparotomy has been associated with decreased mortality (level III).
- In the normothermic patient, on-demand re-laparotomy should be considered with an ongoing transfusion of 2 units of RBC/hour (level III).
- The trends in clinical parameters are predictive of ongoing sepsis or inflammation and failed source control. Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Multiorgan Failure (MOF) score,[6] and Multiorgan Dysfunction score (MODS)[7] can be useful in this regard (level III).
- STAR is indicated or should be considered when there is an inability to eliminate or adequately control the source of infection, incomplete debridement of necrotic tissue, excessive visceral edema, questionable bowel viability, or critical patient condition precluding definitive repair (level III).
- STAR of intra-abdominal injuries should take place after physiologic normalization, (i.e., correction of acidosis, coagulopathy, and hypothermia) optimally within 36 hours or less (level III).
- After STAR, delaying primary fascial closure should be considered in light of intra-abdominal findings (i.e., bowel edema, source control, bowel viability, etc.), and the presence and level of organ dysfunction (level III).
- STAR should be considered after bowel injury with massive fecal contamination or hemorrhagic or septic shock. Staging the gastrointestinal (GI) reconstruction when the patient is hemodynamically stable allows for possible primary bowel anastomosis to be performed with decreased reliance on creating an obligate ostomy (level III).
- After STAR and primary fascial closure, the following should be monitored for early failure of primary fascial closure: (1) renal dysfunction as indicated by an increase in blood urea nitrogen (BUN) and or 20% increase in creatinine on the first post closure day; (2) continued increase in creatinine on the second day post closure; (3) ventilatory disturbances demonstrated by an increase in peak inspiratory pressure and impaired gas exchange; and (4) increased central venous pressure (level III).
Nutrition
- Although direct measurement of abdominal fluid protein loss may be optimal, an estimate of 2 g of nitrogen per liter of abdominal fluid output should be included in the nitrogen balance calculations of any patient with an open abdomen (level II).
- Enteral access and feeding of the patient with an open abdomen with an intact GI tract should be instituted as early as possible, as this may improve the rate of early primary bowel wall closure, fistula formation, and hospital charges (level III).
Scientific Foundation
Indications

Table 3. Indications for the Use of the Open Abdomen Technique in Trauma and Emergency General Surgery

Table 3. Indications for the Use of the Open Abdomen Technique in Trauma and Emergency General Surgery (continued)
Forty-two articles were reviewed with multiple, worldwide indications for the management of the open abdomen (Table 3). More than 1,900 patients were included. The evidence identified is either observational or retrospective in nature, spanning more than 20 years of experience, and as such, it is difficult to link clinical action with event outcome. However, the management of the open abdomen in trauma, transplant, emergency general surgery, and vascular surgery has found a role, and that role seems to be expanding.
Indications have included ACS in its various forms; DC surgery; trauma to include hepatic, severe nonhepatic, and penetrating abdominal trauma, necrotizing pancreatitis, intra-abdominal sepsis, emergent vascular surgery, and recently, orthotropic liver transplantations.
Open Abdomen in the Surgical Management of Abdominal Compartment Syndrome
In 2004, the WCACS met to develop consensus definitions for IAH and ACS.[8][9] These consensus definitions are used to define IAH, primary, secondary, and recurrent ACS. The WCACS definitions have helped further to define the disease processes of IAH and ACS (Tables 1 and 2). ACS is not necessarily an end-stage process, but a continuum of disease, which might be amendable to medical management at an earlier stage. Grade III IAH (IAP >20 mm Hg) should be further monitored with intravesicular pressure monitoring. Medical therapies should be instituted at this point: positioning, negative fluid balance, and drainage of intra-abdominal fluid collections.[10] If these therapies fail to improve IAH or organ dysfunction develops, serious consideration must be given to decompressive laparotomy. Other measures that have been mentioned and that have yet to be studied include neuromuscular blockade, increase sedation, diuresis, evacuation of intraluminal contents, and hemodialysis or hemofiltration in the attempt to decrease IAH.[10]
Abdominal decompression lowers IAH 24.2 ± 9.3 to 14.1 ± 5.5 mm Hg and results in the improvement in lung dynamic compliance from 24.1 ± 7.9 to 27.6 ± 9.4 mL/cm H2O.[11] Abdominal decompression may also be of benefit in the setting of increased intracranial pressure.[12] By using the definition of ACS (the development of significant respiratory compromise, including elevated inspiratory pressure >35 mbar, renal dysfunction [urine <30 mL/hr], hemodynamic instability requiring catecholamines, and a rigid or tense abdomen), it has been found that in these patients, emergency abdominal decompression resulted in a significant increase in the cardiac index, tidal volume, and urine output, with a resultant decrease in bladder pressure, heart rate, central venous pressure, pulmonary artery occlusion pressure, peak airway pressure, partial pressure arterial carbon dioxide, and lactate.[2] Bladder pressures >25 mm Hg have been suggested to indicate ACS.[12] Several studies have demonstrated that ACS may cause a critical increase in the intracranial pressure, which markedly improves after the release of the abdominal tension and aiding in the management of intracranial pressure for patients with traumatic brain injury.[11][13]
Although the weight of the evidence suggests that the open abdomen decreases the risk of ACS, it should be noted that this has not been the universal conclusion of all studies. Raeburn et al.[14] found equivalent results for the development of ACS between fascia closure, skin only and the Bogota bag. Studies attempting to identify the risk factors for ACS suggest that shock, mechanical ventilation, and aggressive fluid resuscitation are common.[14–21] Most studies demonstrate resuscitation volumes of fluid and blood to be much greater in patients with ACS when compared with a randomly selected trauma population who did not develop ACS.[22] Secondary ACS may occur after exsanguination from an extremity injury and when massive volume resuscitation is required. Recurrent ACS occurs after DC in a patient with an open abdomen with either ongoing hemorrhage or massive volume resuscitation. In all scenarios of the ACS, IAP should be monitored.[23–28]
Open Abdomen in the Surgical Management of Penetrating Abdominal Trauma
In patients with penetrating abdominal trauma, TAC with mesh versus primary closure has been advocated when IAH was predicted by lactate level and penetrating abdominal trauma index (PATI). TAC with mesh versus primary closure themselves were also predictive of IAH.[29] Survival improved with open abdomenTAC versus primary closure. IAH developed in 22.2% (10 of 45) of mesh closures and 52% (13 of 25) of primary closures. Lactate level, mesh closure, or its absence, and PATI were the best predictors for IAH on regression analysis.[29] Survival was better in mesh TAC group (40 of 44, 1 excluded secondary to brain death) versus primary closure (17 of 25), p = 0.035. Classic triggers for DC surgery, which have been described in three phases, have been described and may include acidosis with a pH of 7.30, transfusion of 10 or more units of PRBCs (estimated blood loss 4 L), and temperature of 35°C or lower.[16] Survival improved for open abdominal treatment when compared with fascia closure in those with equivalent Injury Severity Score, Revised Trauma Score, Trauma and Injury Severity Score, admission systolic blood pressure, operating room systolic blood pressure, and PATI score.[16][18]
Open Abdomen in the Surgical Management of Severe Hepatic and Nonhepatic Trauma
One early study looked at 35 patients who underwent packing for control of intra-abdominal hemorrhage. The design looked more at packing as a technique, but did some analysis of management of the abdominal wall.[13] Five of 12 patients (42%) closed primarily developed wound infection, compared with 1 of 10 (10%) closed with mesh. Also noted were better peak airway pressures in mesh patients, although these findings were not statistically significant.[30] This preliminary experience supports packing to control coagulopathic bleeding, use of TAC, and further intensive care unit (ICU) resuscitation with a planned second laparotomy for definitive management of GI injuries.[31] These patients with severe nonhepatic injuries shared a constellation of findings including acidosis, hypothermia, and coagulopathy. Protocols to pursue DC should take into account the development of acidosis, hypothermia, and massive transfusion or resuscitation.[32]One suggested protocol established pH of 7.2 or less, temperature 34°C or less, serum bicarbonate level of 15 mEq per liter or less, transfusion volumes of 4000 mL or more of PRBCs, total blood replacement of 5000 mL or more if both PRBCs and whole blood are used, and total operating room fluid replacement of 12,000 mL or more. Groups were compared in a sequential time study before and after protocol. Although mortality was similar between these two groups, the postprotocol group was found to have decreased operative time, transfusions, length of stay, blood loss, infectious complications, and visceral edema.[32]
Open Abdomen in the Surgical Management of Necrotizing Pancreatitis
Descriptive studies have reported salvage rates as high as 80%[16] in small case series of necrotizing pancreatitis in which the open abdomen technique was used, and serial debridement and packing was performed.[11][21][33][34] A retrospective study was performed which included 106 patients who underwent surgical therapy for infected pancreatic necrosis in 11 medical centers in the Netherlands. Surgical approaches included using the OA technique, laparotomy with continuous postoperative lavage, minimally invasive procedures, and laparotomy with primary abdominal closure. Mortality in the open abdomen group was 70% compared with 11% in the minimally invasive group; overall mortality was 34%. The authors concluded that the OA strategy in this patient population should be considered obsolete.[35] Regrettably, this study provided limited criteria for the selection of a particular surgical management strategy (computerized tomography severity index only) and did not account for physiologic severity of illness, rendering it far from definitive. The majority of the literature has described an OA technique with serial laparotomies for patients with necrotizing pancreatitis as safe and effective at decreasing intra-abdominal postoperative infectious complications.[21][36–40] It has been recommended that a regimented reoperative schedule may result in an overall mortality of 19%, which compares favorably to the Dutch study.[36]
Open Abdomen in the Surgical Management of Intra-Abdominal Sepsis
Serial washouts of the open abdomen for peritonitis have been successful in improving the overall morbidity and mortality of the condition.[3–6] One hundred seventeen patients underwent etappenlavage (planned operative take-backs) for peritonitis. On average, six operations were required to control infection. APACHE II predicted mortality was 47%. This group had reduced mortality to 24%. Four different techniques of abdominal closure were used. Certain clinical parameters may suggest which patients have ongoing evidence of sepsis or inflammation after closure of the abdominal wall after planned repeat laparotomy for peritonitis.[41][42] Most patients will have indicators (i.e., renal failure, MOF scores) that source control has not been achieved.[43] Re-exploration will reduce the mortality in these patients. In conclusion, the decision to close the abdomen may not only be based on intraperitoneal findings but also based on the existence and level of organ failure.[43] However, there have been studies that fail to demonstrate a difference in mortality between patients treated with closed versus open abdomen, and demonstrate the reduced mortality in those not undergoing reoperation.[44] It is unclear how these groups compared in acute physiology, but it is reasonable that those successfully treated with one operation and closure had the highest degree of source control at the first operation.[42]
Open Abdomen in Management of Vascular Surgery
Early recognition and delayed abdominal closure has been shown to improve the outcomes in rAAA patients with ACS.[17] The main features of ACS after rAAA were increased central venous pressure (CVP), and mean airway pressure, and low urine output (UOP).[45] The results suggested a decrease in early mortality among patients undergoing delayed abdominal closure. Late mortality because of MOF may also be reduced with delayed abdominal closure.[17] Improved late outcome seems plausible, given the findings of decreased pulmonary damage (improved P/F ratio) and improved tissue oxygenation (SvO2) that was present after early postoperative resuscitation.[15] As in massively resuscitated trauma victims,[16][17][21][29] delayed laparotomy closure in rAAA patients may confer a physiologic and survival benefit. Greater intraoperative blood loss, longer cross clamp times, and longer operative time were risk factors for IAH, which often resulted in colonic ischemia. Earlier decompression and treatment of colonic ischemia may improve mortality.[46] rAAA patients with IAH >21 have a better overall mortality when undergoing abdominal decompression.[15][45]
A small single study used the open abdomen to manage ischemic small bowel after superior mesenteric artery occlusion. OA management proved extremely useful for monitoring blood flow to the anastomotic site and for allowing complete drainage into the abdominal space. Using this method would assist in leaving as much remnant bowel as possible after resection for superior mesenteric artery occlusion.[47]
Temporary Abdominal Closure
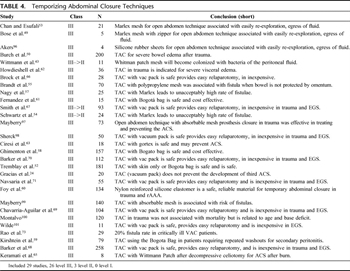
Table 4. Temporizing Abdominal Closure Techniques
As surgeons began managing patients with open abdomens, many techniques were used for TACs (Table 4). The options for TAC are many and include the “Bogotá bag,” fashioned from a large intravenous fluid bag, a ready-to-use transparent “bowel bag,” VP Technique, synthetic mesh (absorbable or non-absorbable), or a Velcro-type sheath as advocated by Wittmann et al.[48] Many have since been abandoned or supplanted by newer techniques. Today, the most common techniques for TAC include the Bogotá Bag, VP, and Wittmann Patch (WP). These methods of closure have wide support in the literature and are considered safe. All allow ready access for relaparotomy procedures and provide a tension-free closure, obviating IAH.
A mesh zipper was reported as one of the earliest methods of TAC[49] in the 1980s. The sterilized zipper was sewn directly to the abdominal fascia. Repeat operations could be performed by removing the outer dressings and unzipping the fascia. Burch et al.[50] reported a large series of critically ill patients who underwent TAC with primary skin closure, towel clip closure, or silo closure (Bogotá Bag) for trauma. At the time, DC procedures were considered highly unorthodox. This series reported a good survival rate for a critically ill group of surgical patients. The complications included skin necrosis, fascial dehiscence, and fascial necrosis. Recognition of the negative effects of forced fascial closure and IAH caused gradual abandonment of the zipper closure technique and replacement with tension-free closure techniques allowing expansion of intra-abdominal contents. Likewise, towel-clip closure of the skin and running suture closure of the skin, while fast and effective, do not allow sufficient fascial expansion to avoid IAH and ACS. These techniques have largely been replaced.[24][51] ACS has been reported to occur in 13%–36% of patients who require DC laparotomy where skin-only or towel clip closure is performed.[14][18][52]
PPE mesh sewn to the fascia to form a fascial bridge was one of the earliest attempts to create a tension-free TAC. Chan and Esufali reported on 21 patients who had PPE placed as a TAC. Ten of the 15 survivors had the mesh removed and were able to undergo primary fascial closure. The five remaining patients had the mesh removed and a split-thickness skin graft applied. No complications resulted from mesh placement.[53] In 1997, Schwartz et al.[54] reported on using PPE with the technical modification of suturing the mesh to the fascia to reduce fascial necrosis. No descriptions of complications were reported. Purported benefits of PPE were its porous nature allowing the egress of fluids as well as low cost. Concerns over a high rate intestinal fistula (7%),[55] 17% to 33%,[56] and 75%,[57] and infection limited general adoption of PPE as a TAC.
Borraez developed the Bogotá bag in 1984. The technique involves sewing a sterile plastic 3-L urologic irrigation bag to the fascia to form a fascial bridge. This technique is simple and inexpensive.[58] It does have the potential for fascial trauma, as it requires sewing to the fascia. It has been used extensively for traumaindications[50] and for abdominal sepsis.[59] Besides intravenous bags, silastic sheeting has been used in a similar manner to the Bogotá bag by a number of authors to achieve a tension-free TAC.[15][17][60–62] This technique is safe and has low incidence of bowel injury and adhesion formation. Eventual fascial closure after this technique, however, is fairly low in most series (28%),[59] and the negative pressure TAC (VP) seems to improve on this.
Gortex (W. L. Gore & Associates, Flagstaff, AZ) mesh has also been used as a fascial bridge for TAC. Nagy et al.[57] reported its use in TAC with no fistula formation. Ciresi[63] reported use of Gortex in patients having laparotomy for trauma and ruptured AAA. The study noted a low rate of reactivity to the Gortex, making re-exploration uncomplicated because of minimal adhesions. The subsequent closure rate was high and fistula rate was very low. The high cost of Gortex, lack of fluid egress, and the potential fascial trauma from suturing the TAC in place have limited the use of Gortex as a TAC.[64]
Use of the WP (Starsurgical, Burlington, WI) was first reported in 1990 for use with serial abdominal washout for severe peritonitis.[48][51] One hundred seventeen patients with abdominal sepsis were prospectively studied. There were no enterocutaneous fistulas reported and no cases of fascial necrosis with the WP when compared with zipper closure or closure with retention sutures. Aprahamian et al.[51]studied the device in 20 consecutive trauma laparotomies. Fifteen of the 16 survivors underwent primary fascial closure at subsequent operation. In one patient, the device was removed due to fascial infection requiring surgical debridement. In a small series of patients developing ACS, WP was associated with no complications, and all survivors were able to undergo primary fascial closure.[65]
The WP consists of two sheets of hook-and-burr material (similar to Velcro) that is sewn to the fascial edges after a plastic drape is placed over the viscera. The hook-and-burr are then overlapped with limited tension to provide a secure TAC. Gauze is used to pack the subcutaneous tissue.[48] Pulling the Velcro-like material apart easily allows for re-exploration of the abdomen. At the completion of the subsequent operations, the patch can be tightened to keep fascial tension. Repeated tightening of the patch allows for a gradual sequential closure of the fascia.
Brock et al.[66] first reported the VP technique in 1995. A larger study with a combined population of traumaand emergent general surgical patients was reported in 1997.[67] The majority of these patients were able to undergo primary fascial closure at the time of their second laparotomy. Fascial closure rate was 71% for the emergency general surgery (EGS) group and 61% for the trauma group. Barker et al.[68] reported a 68% fascial closure rate in a combined population. The same group reported their experience using VP with destructive bowel injuries requiring resection.[69] No difference in fistula or anastomotic leak was noted between patients having VP or other types of TAC. The reported fistula rate in the larger studies is 3% to 5%.[70][71] This technique has proven to be efficient and allows for safe movement of the patient and prone positioning of the patient if needed.[24]
The VP technique uses a three-layer TAC. First, a fenestrated polyvinyl sheet (ISO 1010 Drape, Microtek Medical, Columbus, MS) is draped over the exposed viscera and tucked under the fascial edges. Next, a surgical towel is placed under the fascia followed by two silicone drains (Jackson-Pratt Drain, Allegiance, McGaw Park, IL), which are placed on top of the towel. An adhesive, iodophor-impregnated polyester drape (Ioban 2, 3 mol/L Healthcare, St. Paul, MN) is placed over the skin laterally to the anterior axillary lines to seal the wound. The surgical drains are connected to a Y-connector, and wall suction is applied. This dressing has gained wide acceptance because it is fast to apply, inexpensive, atraumatic and allows for excellent control of abdominal fluids. It is also cost effective at approximately $50 per application.[68][70] VP remains the most popular TAC used today for trauma and emergent general surgery. It is the current standard of care for TAC.
A commercial version of the VP has been performed using the VAC Abdominal Dressing System from KCI (San Antonio, TX). There have been a number of previous reports that have highlighted the advantages of VAC therapy as TAC. In a series of 112 patients, 11 (9.8%) developed abdominal complications, of whom five (4.5%) developed a fistula: three from the small bowel, one pancreatic, and one gastric. Two of the small bowel fistulae and the gastric fistula occurred in patients who had an intestinal resection and anastomosis at their primary operation.[70] Miller and coworkers[72] reported the use of VAC in 45 patients with minimal complications and a 48% primary fascial closure rate. One series, with 29 emergent general surgical patients requiring bowel resection, reported a very high incidence (20%) of enterocutaneous fistulas.[73]
The ideal TAC would fulfill the following criteria: easy to apply, tension free, atraumatic, inexpensive, and allow for a high rate of definitive fascial closure when the device is no longer needed. Currently, the most popular techniques of TAC are the VP, WP, and the Bogotá bag. All these techniques are safe and allow ready access for relaparotomy. They are also tension free, thus avoiding the added complication of IAH and ACS. VP has the added advantage of not needing to be sutured to the fascia, saving time, and potential tissue destruction. There does not seem to be a single TAC that is superior to the others commonly in use. It is largely a matter of surgeon preference, and without direct comparison of the commonly used techniques a single method cannot be recommended.
Relaparotomy/Staged Abdominal Reconstruction
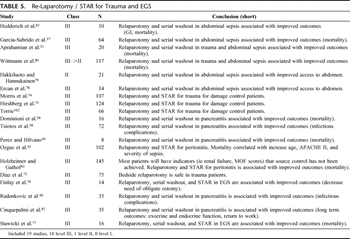
Table 5. Re-Laparotomy / STAR for Trauma and EGS
Relaparotomy and STAR serves three main functions: washout to reduce contamination and control intra-abdominal sepsis, resection or debridement of devitalized or contaminated tissue, and reconstruction of the GI tract (Table 5). Relaparotomy and STAR should be performed when the patient has been adequately resuscitated as demonstrated by correction of hypothermia, acidosis, and coagulopathy.[74] This can usually be accomplished within 36 hours.[74] This technique has been shown to improve the outcomes in severely injured trauma patients.[75]
An ideal setting for reconstruction of internal injuries must be achieved.[42][43] Injured devitalized tissue is resected, and GI injuries can be anastomosed safely, mitigating the necessity for obligate ostomy. However, in high risk patients, ostomy remains the most conservative approach.[76] Relaparotomy with routinely scheduled abdominal washout has been used as a means to effectively manage the patient with severe intra-abdominal sepsis. It has been well tolerated with few GI complications. Several authors have suggested decreased mortality.[42][77–81] Utilization in severe necrotizing pancreatitis, in general, is associated with improved mortality, although there are mixed results in less severe cases.[36–40][51][82]
In the patient with sepsis, the clinical parameters such as renal dysfunction, APACHE II score, and MODS score were predictive of on going intra-abdominal sepsis[6][7][83] and were the indications for relaparotomy.[43][72][76][77][80] Those patients with continued intra-abdominal sepsis who underwent repeat laparotomy had reduced mortality.[43] In patients with high ventilatory demands, bedside relaparotomy has provided a safe adjunct with risks similar to those performed in the operative theater.[72]
A clear benefit for planned relaparotomy versus on demand has not been demonstrated.[84] Van Ruler et al. in a randomized controlled trial of 116 on-demand and 116 planned relaparotomies in the setting of peritonitis demonstrated no significant difference in primary end point (57% on-demand [n = 64] vs. 65% planned [n = 73], p = 0.25) or in mortality or morbidity alone (29% on-demand [n = 32] vs. 36% planned [n = 41], p = 0.22) (40% on-demand [n = 32] vs. 44% planned [n = 32], p = 0.58), respectively. A total of 42% of the on-demand patients had a relaparotomy versus 94% of the planned relaparotomy group. Thirty-one percent of first relaparotomies were negative in the on-demand group versus 66% in the planned group (p = 0.001). Patients in the on-demand group had shorter median ICU stays (7 vs. 11 days, p = 0.001) and shorter median hospital stays (27 vs. 35 days, p = 0.008). Direct medical costs per patient were reduced by 23% using the on-demand strategy. On-demand relaparotomy did not have a significantly lower rate of death or major peritonitis-related morbidity compared with the planned relaparotomy group but did have a substantial reduction in relaparotomies, healthcare utilization, and medical costs.[84]
Nutrition Support of the Open Abdomen
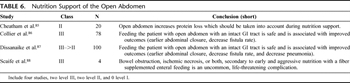
Table 6. Nutrition Support of the Open Abdomen
Early nutritional support is well described in surgical literature. Evidence that it is safe, well tolerated, decreases hospital length of stay, and may reduce infectious complications is clear. However, the idea of early enteral nutrition in the management of the open abdomen is relatively poorly investigated (Table 6). We can infer from a relatively recent study that the OA represents a significant source of protein or nitrogen loss in the critically ill. Failure to account for this loss in nutritional calculations may lead to underfeeding and inadequate nutritional support with a negative effect on patient outcome. Although direct measurement of abdominal fluid protein loss may be optimal, an estimate of 2 g of nitrogen per liter of abdominal fluid output should be included in the nitrogen balance calculations of any patient with an open abdomen.[85]
Furthermore, early enteral nutrition (<4 days) is well tolerated, and in comparison with delayed enteral nutrition may result in higher primary fascial closure (74% vs. 49%; p = 0.02), lower fistula rate (9% vs. 26%; p = 0.05) and lower total hospital charges.[86] Early enteral nutrition instituted in less than 48 hours is well tolerated in open abdomens for trauma and reduces nosocomial infections (most notably pneumonia) with no significant difference in multiorgan dysfunction syndrome, length of ventilator days, ICU days, hospital days, or mortality.[87] Although these results are encouraging, in the absence of a larger body of literature, any recommendation must be made with caution, and further study is necessary to make significant inferences.
A special note should be made of the extremely rare occurrence of nonocclusive bowel ischemia because of early and aggressive enteral feeding.[88] Tube feedings should be discontinued immediately, and total parenteral nutrition (TPN) should be started in patients with abdominal pain, distension, increased nasogastric drainage, and signs of intestinal ileus.[89] Laparotomy should be considered in patients who manifest an acute surgical abdomen.
Conclusion
Through its various evolutions, the techniques of OA management have demonstrated usefulness in surgery. From life-saving decompression of ACS in vascular surgery and DC to providing ready and repeated access for source control in abdominal sepsis, the last 30 years have provided a substantial body of clinical experience to guide our endeavor to decrease morbidity and mortality. There remains a great degree of heterogeneity in the patient populations and the surgical techniques described. We hope these recommendations provide a means to guide the indications, use, and early management of open abdomenin both trauma and nontrauma surgery.
References
- Ogilvie WH. The late complications of abdominal war-wounds. Lancet. 1940;2:253–256. CrossRef
- Stone HH, Lamb JM. Use of pedicled omentum as an autogenous pack for control of hemorrhage in major injuries of the liver. Surg Gynecol Obstet. 1975;141:92–94. PubMed
- Stone HH, Strom PR, Mullins RJ. Management of the major coagulopathy with onset during laparotomy. Ann Surg. 1983;197:532–535. View Full Text| PubMed| CrossRef
- Lucas CE, Ledgerwood AM. Prospective evaluation of hemostatic techniques for liver injuries. J Trauma. 1976;16:442–451. View Full Text| PubMed| CrossRef
- Rotondo MF, Schwab CW, McGonigal MD, et al. ‘Damage control’: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35:375–382; discussion 382–383.
- Goris RJ, Nuytinck HK, Redl H. Scoring systems and predictors of ARDS and MOF. Prog Clin Biol Res. 1987;236:3–15. PubMed
- Marshall JC. SIRS and MODS: what is their relevance to the science and practice of intensive care? Shock. 2000;14:586–589. View Full Text| PubMed| CrossRef
- Cheatham ML, Malbrain ML, Kirkpatrick A, et al. Results from the International Conference of Experts on intra-abdominal hypertension and abdominal compartment syndrome. II. Recommendations. Intensive Care Med. 2007;33:951–962. View Full Text| PubMed| CrossRef
- Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. I. Definitions. Intensive Care Med. 2006;32:1722–1732. View Full Text| PubMed| CrossRef
- Cheatham ML. Nonoperative management of intraabdominal hypertension and abdominal compartment syndrome. World J Surg. 2009;33:1116–1122. PubMed| CrossRef
- Sugrue M, Jones F, Janjua KJ, et al. Temporary abdominal closure: a prospective evaluation of its effects on renal and respiratory physiology [see comment]. J Trauma. 1998;45:914–921. View Full Text| PubMed| CrossRef
- Ertel W, Oberholzer A, Platz A, Stocker R, Trentz O. Incidence and clinical pattern of the abdominal compartment syndrome after “damage-control” laparotomy in 311 patients with severe abdominal and/or pelvic trauma. Crit Care Med. 2000;28:1747–1753. View Full Text| PubMed| CrossRef
- Scalea TM, Bochicchio GV, Habashi N, et al. Increased intra-abdominal, intrathoracic, and intracranial pressure after severe brain injury: multiple compartment syndrome. J Trauma. 2007;62:647–656; discussion 656.
- Raeburn CD, Moore EE, Biffl WL, et al. The abdominal compartment syndrome is a morbid complication of postinjury damage control surgery [see comment]. Am J Surg. 2001;182:542–546. PubMed| CrossRef
- Oelschlager BK, Boyle EM Jr, Johansen K, Meissner MH. Delayed abdominal closure in the management of ruptured abdominal aortic aneurysms. Am J Surg. 1997;173:411–415. PubMed| CrossRef
- Johnson JW, Gracias VH, Schwab CW, et al. Evolution in damage control for exsanguinating penetrating abdominal injury. J Trauma. 2001;51:261–269; discussion 269–271.
- Rasmussen TE, Hallett JW Jr, Noel AA, et al. Early abdominal closure with mesh reduces multiple organ failure after ruptured abdominal aortic aneurysm repair: guidelines from a 10-year case-control study. J Vasc Surg. 2002;35:246–253. View Full Text| PubMed| CrossRef
- Offner PJ, de Souza AL, Moore EE, et al. Avoidance of abdominal compartment syndrome in damage-control laparotomy after trauma. Arch Surg. 2001;136:676–681. View Full Text| PubMed| CrossRef
- Nicholas JM, Rix EP, Easley KA, et al. Changing patterns in the management of penetrating abdominal trauma: the more things change, the more they stay the same. J Trauma. 2003;55:1095–1108; discussion 1108–1010.
- Mayberry JC, Welker KJ, Goldman RK, Mullins RJ. Mechanism of acute ascites formation after traumaresuscitation. Arch Surg. 2003;138:773–776. View Full Text| PubMed| CrossRef
- De Waele JJ, Hoste E, Blot SI, Decruyenaere J, Colardyn F. Intra-abdominal hypertension in patients with severe acute pancreatitis. Crit Care. 2005;9:R452–R457. PubMed| CrossRef
- Rodas EB, Malhotra AK, Chhitwal R, Aboutanos MB, Duane TM, Ivatury RR. Hyperacute abdominal compartment syndrome: an unrecognized complication of massive intraoperative resuscitation for extra-abdominal injuries. Am Surg. 2005;71:977–981. PubMed
- Balogh Z, McKinley BA, Cocanour CS, et al. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg. 2003;138:637–642; discussion 642–643.
- Gracias VH, Braslow B, Johnson J, et al. Abdominal compartment syndrome in the open abdomen. Arch Surg. 2002;137:1298–1300. View Full Text| PubMed| CrossRef
- O'Mara MS, Slater H, Goldfarb IW, Caushaj PF. A prospective, randomized evaluation of intra-abdominal pressures with crystalloid and colloid resuscitation in burn patients. J Trauma. 2005;58:1011–1018. View Full Text| PubMed| CrossRef
- Balogh Z, McKinley BA, Holcomb JB, et al. Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J Trauma. 2003;54:848–859; discussion 859–861.
- Balogh Z, McKinley BA, Cocanour CS, et al. Secondary abdominal compartment syndrome is an elusive early complication of traumatic shock resuscitation. Am J Surg. 2002;184:538–543; discussion 543–544.
- Biffl WL, Moore EE, Burch JM, Offner PJ, Franciose RJ, Johnson JL. Secondary abdominal compartment syndrome is a highly lethal event. Am J Surg. 2001;182:645–648. PubMed| CrossRef
- Ivatury RR, Porter JM, Simon RJ, Islam S, John R, Stahl WM. Intra-abdominal hypertension after life-threatening penetrating abdominal trauma: prophylaxis, incidence, and clinical relevance to gastric mucosal pH and abdominal compartment syndrome. J Trauma. 1998;44:1016–1021; discussion 1021–1023.
- Cue JI, Cryer HG, Miller FB, Richardson JD, Polk HC Jr. Packing and planned reexploration for hepatic and retroperitoneal hemorrhage: critical refinements of a useful technique. J Trauma. 1990;30:1007–1011; discussion 1011–1013.
- Talbert S, Trooskin SZ, Scalea T, et al. Packing and re-exploration for patients with nonhepatic injuries. J Trauma. 1992;33:121–124; discussion 124–125.
- Asensio JA, Petrone P, Roldan G, Kuncir E, Ramicone E, Chan L. Has evolution in awareness of guidelines for institution of damage control improved outcome in the management of the posttraumatic open abdomen? Arch Surg. 2004;139:209–214; discussion 215.
- Wertheimer MD, Norris CS. Surgical management of necrotizing pancreatitis. Arch Surg. 1986;121:484–487. PubMed| CrossRef
- Agalar F, Eroglu E, Bulbul M, Agalar C, Tarhan OR, Sari M. Staged abdominal repair for treatment of moderate to severe secondary peritonitis. World J Surg. 2005;29:240–244. PubMed| CrossRef
- Besselink MG, de Bruijn MT, Rutten JP, et al. Surgical intervention in patients with necrotizing pancreatitis. Br J Surg. 2006;93:593–599. View Full Text| PubMed| CrossRef
- Dominioni L, Chiappa A, Bianchi V, et al. Infected pancreatic necrosis complicated by multiple organ failure. Hepatogastroenterology. 1997;44:968–974. PubMed
- Garcia-Sabrido JL, Tallado JM, Christou NV, Polo JR, Valdecantos E. Treatment of severe intra-abdominal sepsis and/or necrotic foci by an ‘open-abdomen’ approach. Zipper and zipper-mesh techniques. Arch Surg. 1988;123:152–156. PubMed| CrossRef
- Tsiotos GG, Luque-de Leon E, Soreide JA, et al. Management of necrotizing pancreatitis by repeated operative necrosectomy using a zipper technique. Am J Surg. 1998;175:91–98. PubMed| CrossRef
- Perez A, Hilvano S. Abdominal zippers for temporary abdominal closure in planned relaparotomies for peripancreatic sepsis: experience in a developing country. J Hepatobiliary Pancreat Surg. 2001;8:449–452. PubMed| CrossRef
- Radenkovic DV, Bajec DD, Tsiotos GG, et al. Planned staged reoperative necrosectomy using an abdominal zipper in the treatment of necrotizing pancreatitis. Surg Today. 2005;35:833–840. PubMed| CrossRef
- Ivatury RR, Nallathambi M, Rao PM, Rohman M, Stahl WM. Open management of the septic abdomen: therapeutic and prognostic considerations based on APACHE II. Crit Care Med. 1989;17:511–517. View Full Text| PubMed| CrossRef
- Ozguc H, Yilmazlar T, Gurluler E, Ozen Y, Korun N, Zorluoglu A. Staged abdominal repair in the treatment of intra-abdominal infection: analysis of 102 patients. J Gastrointest Surg. 2003;7:646–651. PubMed| CrossRef
- Holzheimer RG, Gathof B. Re-operation for complicated secondary peritonitis—how to identify patients at risk for persistent sepsis. Eur J Med Res. 2003;8:125–134. PubMed
- Adkins AL, Robbins J, Villalba M, Bendick P, Shanley CJ. Open abdomen management of intra-abdominal sepsis. Am Surg. 2004;70:137–140; discussion 140.
- Fietsam R Jr, Villalba M, Glover JL, Clark K. Intra-abdominal compartment syndrome as a complication of ruptured abdominal aortic aneurysm repair. Am Surg. 1989;55:396–402. PubMed
- Djavani K, Wanhainen A, Bjorck M. Intra-abdominal hypertension and abdominal compartment syndrome following surgery for ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2006;31:581–584. PubMed| CrossRef
- Mimatsu K, Oida T, Kanou H, Miyake H, Amano S. Open abdomen management after massive bowel resection for superior mesenteric arterial occlusion. Surg Today. 2006;36:241–244. PubMed| CrossRef
- Wittmann DH, Aprahamian C, Bergstein JM, et al. A burr-like device to facilitate temporary abdominal closure in planned multiple laparotomies. Eur J Surg. 1993;159:75–79. PubMed
- Bose SM, Kalra M, Sandhu NP. Open management of septic abdomen by Marlex mesh zipper. Aust N Z J Surg. 1991;61:385–388. PubMed
- Burch JM, Ortiz VB, Richardson RJ, Martin RR, Mattox KL, Jordan GL Jr. Abbreviated laparotomy and planned reoperation for critically injured patients. Ann Surg. 1992;215:476–483; discussion 483–484.
- Aprahamian C, Wittmann DH, Bergstein JM, Quebbeman EJ. Temporary abdominal closure (TAC) for planned relaparotomy (etappenlavage) in trauma. J Trauma. 1990;30:719–723. View Full Text| PubMed| CrossRef
- Tremblay LN, Feliciano DV, Schmidt J, et al. Skin only or silo closure in the critically ill patient with an open abdomen. Am J Surg. 2001;182:670–675. PubMed| CrossRef
- Chan ST, Esufali ST. Extended indications for polypropylene mesh closure of the abdominal wall. Br J Surg. 1986;73:3–6. PubMed| CrossRef
- Schwartz A, Onaca N, Rabi I, Bass A. Closure of the abdomen by mesh for planned re-laparotomy. A technical modification. Int Surg. 1997;82:42–43. PubMed
- Brandt CP, McHenry CR, Jacobs DG, Piotrowski JJ, Priebe PP. Polypropylene mesh closure after emergency laparotomy: morbidity and outcome. Surgery. 1995;118:736–740; discussion 740–741.
- Voyles CR, Richardson JD, Bland KI, Tobin GR, Flint LM, Polk HC Jr. Emergency abdominal wall reconstruction with polypropylene mesh: short-term benefits versus long-term complications. Ann Surg. 1981;194:219–223. View Full Text| PubMed| CrossRef
- Nagy KK, Fildes JJ, Mahr C, et al. Experience with three prosthetic materials in temporary abdominal wall closure [see comment]. Am Surg. 1996;62:331–335. PubMed
- Ghimenton F, Thomson SR, Muckart DJ, Burrows R. Abdominal content containment: practicalities and outcome. Br J Surg. 2000;87:106–109. View Full Text| PubMed| CrossRef
- Kirshtein B, Roy-Shapira A, Lantsberg L, Mizrahi S. Use of the “Bogota bag” for temporary abdominal closure in patients with secondary peritonitis. Am Surg. 2007;73:249–252. PubMed
- Foy HM, Nathens AB, Maser B, Mathur S, Jurkovich GJ. Reinforced silicone elastomer sheeting, an improved method of temporary abdominal closure in damage control laparotomy. Am J Surg. 2003;185:498–501. PubMed| CrossRef
- Fernandez L, Norwood S, Roettger R, Wilkins HE III. Temporary intravenous bag silo closure in severe abdominal trauma. J Trauma. 1996;40:258–260. View Full Text| PubMed| CrossRef
- Howdieshell TR, Yeh KA, Hawkins ML, Cué JI. Temporary abdominal wall closure in trauma patients: indications, technique, and results. World J Surg. 1995;19:154–158; discussion 158.
- Ciresi DL, Cali RF, Senagore AJ. Abdominal closure using nonabsorbable mesh after massive resuscitation prevents abdominal compartment syndrome and gastrointestinal fistula. Am Surg. 1999;65:720–724; discussion 724–725.
- Vertrees A, Greer L, Pickett C, et al. Modern management of complex open abdominal wounds of war: a 5-year experience. J Am Coll Surg. 2008;207:801–809. PubMed| CrossRef
- Keramati M, Srivastava A, Sakabu S, et al. The Wittmann Patch s a temporary abdominal closure device after decompressive celiotomy for abdominal compartment syndrome following burn. Burns. 2008;34:493–497. PubMed| CrossRef
- Brock WB, Barker DE, Burns RP. Temporary closure of open abdominal wounds: the vacuum pack. Am Surg. 1995;61:30–35. PubMed
- Smith LA, Barker DE, Chase CW, Somberg LB, Brock WB, Burns RP. Vacuum pack technique of temporary abdominal closure: a four-year experience. Am Surg. 1997;63:1102–1107; discussion 1107–1108.
- Barker DE, Green JM, Maxwell RA, et al. Experience with vacuum-pack temporary abdominal wound closure in 258 trauma and general and vascular surgical patients. J Am Coll Surg. 2007;204:784–792; discussion 792–793.
- Chavarria-Aguilar M, Cockerham WT, Barker DE, Ciraulo DL, Richart CM, Maxwell RA. Management of destructive bowel injury in the open abdomen. J Trauma. 2004;56:560–564. View Full Text| PubMed| CrossRef
- Barker DE, Kaufman HJ, Smith LA, Ciraulo DL, Richart CL, Burns RP. Vacuum pack technique of temporary abdominal closure: a 7-year experience with 112 patients. J Trauma. 2000;48:201–206; discussion 206–207.
- Navsaria PH, Bunting M, Omoshoro-Jones J, Nicol AJ, Kahn D. Temporary closure of open abdominal wounds by the modified sandwich-vacuum pack technique [see comment]. Br J Surg. 2003;90:718–722. View Full Text| PubMed| CrossRef
- Diaz JJ Jr, Mauer A, May AK, Miller R, Guy JS, Morris JA Jr. Bedside laparotomy for trauma: are there risks? Surg Infect (Larchmt). 2004;5:15–20. PubMed| CrossRef
- Rao M, Burke D, Finan PJ, Sagar PM. The use of vacuum-assisted closure of abdominal wounds: a word of caution [see comment]. Colorectal Dis. 2007;9:266–268. View Full Text| PubMed| CrossRef
- Morris JA Jr, Eddy VA, Blinman TA, Rutherford EJ, Sharp KW. The staged celiotomy for trauma. Issues in unpacking and reconstruction. Ann Surg. 1993;217:576–584; discussion 584–586.
- Hirshberg A, Wall MJ Jr, Mattox KL. Planned reoperation for trauma: a two year experience with 124 consecutive patients. J Trauma. 1994;37:365–369. View Full Text| PubMed| CrossRef
- Finlay IG, Edwards TJ, Lambert AW. Damage control laparotomy. Br J Surg. 2004;91:83–85. View Full Text| PubMed| CrossRef
- Stawicki SP, Brooks A, Bilski T, et al. The concept of damage control: extending the paradigm to emergency general surgery. Injury. 2008;39:93–101. PubMed| CrossRef
- Ercan F, Korkmaz A, Aras N. The zipper-mesh method for treating delayed generalized peritonitis. Surg Today. 1993;23:205–214. PubMed| CrossRef
- Hakkiluoto A, Hannukainen J. Open management with mesh and zipper of patients with intra-abdominal abscesses or diffuse peritonitis. Eur J Surg. 1992;158:403–405. PubMed
- Wittmann DH, Aprahamian C, Bergstein JM. Etappenlavage: advanced diffuse peritonitis managed by planned multiple laparotomies utilizing zippers, slide fastener, and Velcro analogue for temporary abdominal closure. World J Surg. 1990;14:218–226. PubMed| CrossRef
- Hedderich GS, Wexler MJ, McLean AP, Meakins JL. The septic abdomen: open management with Marlex mesh with a zipper. Surgery. 1986;99:399–408. PubMed
- Cinquepalmi L, Boni L, Dionigi G, et al. Long-term results and quality of life of patients undergoing sequential surgical treatment for severe acute pancreatitis complicated by infected pancreatic necrosis. Surg Infect (Larchmt). 2006;7(Suppl 2):S113–S116.
- Goris RJ. Mediators of multiple organ failure. Intensive Care Med. 1990;16(Suppl 3):S192–S196. PubMed| CrossRef
- van Ruler O, Mahler CW, Boer KR, et al. Comparison of on-demand vs planned relaparotomy strategy in patients with severe peritonitis: a randomized trial. JAMA. 2007;298:865–872. View Full Text| PubMed| CrossRef
- Cheatham ML, Safcsak K, Brzezinski SJ, Lube MW. Nitrogen balance, protein loss, and the open abdomen [see comment]. Crit Care Med. 2007;35:127–131. View Full Text| PubMed| CrossRef
- Collier B, Guillamondegui O, Cotton B, et al. Feeding the open abdomen. PEN J Parenter Enteral Nutr. 2007;31:410–415.
- Dissanaike S, Pham T, Shalhub S, et al. Effect of immediate enteral feeding on trauma patients with an open abdomen: protection from nosocomial infections. J Am Coll Surg. 2008;207:690–697. PubMed| CrossRef
- Scaife CL, Saffle JR, Morris SE. Intestinal obstruction secondary to enteral feedings in burn trauma patients. J Trauma. 1999;47:859–863. View Full Text| PubMed| CrossRef
- Schunn CD, Daly JM. Small bowel necrosis associated with postoperative jejunal tube feeding. J Am Coll Surg. 1995;180:410–416. PubMed
- Walsh GL, Chiasson P, Hedderich G, et al. The open abdomen. The Marlex mesh and zipper technique: a method of managing intraperitoneal infection. Surgical Clinics of North America. 1988;68:25–40. PubMed| CrossRef
- Cuesta MA, Doblas M, Castaneda L, et al. Sequential abdominal reexploration with the zipper technique. World Journal of Surgery. 1991;15:74–80. PubMed| CrossRef
- Arthurs Z, Kjorstad R, Mullenix P, et al. The use of damage-control principles for penetrating pelvic battlefield trauma. American Journal of Surgery. 2006;191:604–609. PubMed| CrossRef
- Jafri MA, Tevar AD, Lucia M, et al. Temporary silastic mesh closure for adult liver transplantation: a safe alternative for the difficult abdomen. [see comment]. Liver Transplantation. 2007;13:258–265. View Full Text| PubMed| CrossRef
- Maxwell RA, Fabian TC, Croce MA, et al. Secondary abdominal compartment syndrome: an underappreciated manifestation of severe hemorrhagic shock. J Trauma. 1999;47:995–999. View Full Text| PubMed| CrossRef
- Latenser BA, Kowal-Vern A, Kimball D, et al. A pilot study comparing percutaneous decompression with decompressive laparotomy for acute abdominal compartment syndrome in thermal injury. J Burn Care Rehabil. 2002;23:190–195. View Full Text| PubMed| CrossRef
- Akers DL Jr, Fowl RJ, Kempczinski RF, et al. Temporary closure of the abdominal wall by use of silicone rubber sheets after operative repair of ruptured abdominal aortic aneurysms. Journal of Vascular Surgery. 1991;14:48–52. PubMed| CrossRef
- Mayberry JC, Mullins RJ, Crass RA, et al. Prevention of abdominal compartment syndrome by absorbable mesh prosthesis closure. [see comment]. Archives of Surgery. 1997;132:957–961; discussion 952–961.
- Sherck J, Seiver A, Shatney C, et al. Covering the “open abdomen”: a better technique. [erratum appears in Am Surg. 1999 Jan;65(1):98]. American Surgeon. 1998;64:854–857. PubMed
- Mayberry JC, Burgess EA, Goldman RK, et al. Enterocutaneous fistula and ventral hernia after absorbable mesh prosthesis closure for trauma: the plain truth. Journal of Trauma-Injury Infection & Critical Care. 2004;57:157–162; discussion 153–163.
- Montalvo JA, Acosta JA, Rodriguez P, et al. Surgical complications and causes of death in trauma patients that require temporary abdominal closure. American Surgeon. 2005;71:219–224. PubMed
- Wilde JM, Loudon MA. Modified Opsite sandwich for temporary abdominal closure: a non-traumatic experience. Annals of the Royal College of Surgeons of England. 2007;89:57–61. View Full Text| PubMed| CrossRef
- Torrie J, Hill AA, Streat S. Staged abdominal repair in critical illness. Anaesthesia & Intensive Care. 1996;24:368–374. PubMed
Editorial Comment
Diaz et al.[1] have done a superb job of analyzing the complex, heterogenous literature on damage control. These practice management guidelines (PMG) measure up to the high standards of others created by the Eastern Association for the Surgery of Trauma (EAST).
The EAST Primer of 2000 on PMG development[2] has become the beacon that directs us to advance recommendations at levels I to III, based on evidence as classes I to III. However, it is clear from a review of the various PMG of EAST that class I evidence (prospective, randomized trials) exists for few clinical problems. Their solutions must necessarily be based on class II or even class III evidence. The current PMG are no exception. Only one report of the 95 relevant articles reviewed, on burn resuscitation with colloids versus crystalloids, had the strength of a class I evidence but was not strong enough to warrant a level I recommendation.
There was only one level I recommendation in the current PMG, relating to emergent/urgent decompressive laparotomy for abdominal compartment syndrome, as defined by consensus opinion of the World Congress of Abdominal Compartment Syndrome. Expert opinion and consensus panel discussions have not been given much eminence by the EAST primer. Nevertheless, the authors to their credit recognized their value and included them in their analysis to form many level II and a few level III recommendations on intraabdominal hypertension and abdominal compartment syndrome. They proved to be prescient: in a report too recent to be included in this analysis, Cheatham and Safcsak[3] documented improved survival, reduced resource utilization, and increased fascial closure by management protocols refined by algorithms and definitions of the World Society of Abdominal Compartment Syndrome. The accompanying editorial[4]applauded: “the waiting is over: the first clinical outcome study of the treatment of intra-abdominal hypertension has arrived.”
Consensus opinion that is a product of rigorous analysis and discussion, in concert with even lowly class III data, may lead to strong level II recommendations as shown by these PMG. The importance of this process cannot be overstated: first, they emphasize the benefits of temporary abdominal closure and open abdomen management. Second, they collate current refinements in our management of these critically injured or ill patients and the role of prevention, monitoring, and prompt treatment of intraabdominal hypertension. Third, they serve to promulgate the current knowledge about these preventable complications and help rectify the obstinate “never” and “do-not-believe-in-it” attitudes of clinicians still prevalent in different countries, specialties of critical care, and even specialties of surgery.[5] In the interest of full disclosure, this writer is a member and an officer of the executive committee of World Society of Abdominal Compartment Syndrome.
Rao R. Ivatury, MD
Department of Surgery
Medical College of Virginia Hospitals
Richmond, Virginia
Editorial References
- Diaz JJ, Cullinanae DC, Dutton WD, et al. Open abdomen in trauma and emergency general surgery: part 1 “damage control.” J Trauma. 2010;68:1425–1438. View Full Text| PubMed| CrossRef
- Eastern Association for the Surgery of Trauma. Utilizing evidence based outcome measures to develop practice management guidelines: a primer. Eastern Association for the Surgery of Trauma (EAST) Ad Hoc Committee on Practice Management Guideline Development. 2000. Available at: www.EAST.org. Accessed February 2, 2010.
- Cheatham ML, Safcsak K. Is the evolving management of intra-abdominal hypertension and abdominal compartment syndrome improving survival? Crit Care Med. 2010;38:402–408. View Full Text| PubMed| CrossRef
- Sriram K, Mizock BA. The waiting is over: the first clinical outcome study of the treatment of intraabdominal hypertension has arrived. Crit Care Med. 2010;38:692–693.
- Ivatury RR. Abdominal compartment syndrome: a century later, isn't it time to accept and promulgate? Crit Care Med. 2006;34:2494–2495. View Full Text| PubMed| CrossRef
Keywords:
Open abdomen; Trauma; Damage control; Temporary abdominal closure; Emergency general surgery; Emergency vascular surgery; Acute pancreatitis; Intraabdominal sepsis; Staged abdominal reconstruction (STAR); Nutrition in trauma