Open Abdomen Management, A Review: Part 2
Published 2011
Citation: J Trauma. 71(2):502-512, August 2011
Authors
Diaz, Jose J. Jr. MD; Dutton, William D. MD; Ott, Mickey M. MD; Cullinane, Daniel C. MD; Alouidor, Reginald MD; Armen, Scott B. MD; Bilanuik, Jaroslaw W. MD; Collier, Bryan R. DO; Gunter, Oliver L. MD; Jawa, Randeep MD; Jerome, Rebecca MS; Kerwin, Andrew J. MD; Kirby, John P. MD; Lambert, Anne L. MD; Riordan, William P. MD; Wohltmann, Christopher D. MD
Author Information
From the Division of Trauma and Surgical Critical Care (J.J.D., W.D.D., M.M.O., B.R.C., O.L.G., W.P.R.), Department of Surgery, and Department of Biomedical Informatics (R.J.), Vanderbilt University Medical Center, Nashville, Tennessee; Division of Trauma, Critical Care, and General Surgery (D.C.C.), Department of Surgery, Mayo Clinic, Rochester, Minnesota; Division of Trauma, Department of Surgery (R.A.), Bay State Medical Center, Springfield, Massachusetts; Division of Trauma, Acute Care & Critical Care Surgery (S.B.A.), Penn State Hershey Medical Center, Hershey, Pennsylvania; Department of Surgery (J.W.B.), Morristown Memorial Hospital, Morristown, New Jersey; Department of Surgery (R.J.), University of Nebraska Medical Center, Omaha, Nebraska; Division of Acute Care Surgery, Department of Surgery (A.J.K.), University of Florida HSC, Jacksonville, Florida; Division of General Surgery (J.P.K.), Section of Acute and Critical Care Surgery, Washington University Medical Center, St. Louis, Missouri; Department of Surgery - General/Trauma and Plastic, Burn, & Reconstructive Surgery (A.L.L.), Hennepin County Medical Center, Health Partners/Regions Health, St. Paul, Minnesota; and Division of General Surgery (C.D.W.), Department of Surgery, Southern Illinois University School of Medicine, Springfield, Illinois.
Submitted for publication July 21, 2010.
Accepted for publication May 31, 2011.
Address for reprints: Jose J. Diaz, Jr., MD, CNS, FACS, FCCM, Division of Trauma and Surgical Critical Care, Department of Surgery, Vanderbilt University Medical Center, 1211 21st Avenue South, 404 Medical Arts Building, Nashville, TN 37212; email: jose.diaz@vanderbilt.edu.
Overview
During the course of the last 30 years, several authors have contributed their clinical experience to the literature in an effort to describe the various management strategies for the appropriate use of the open abdomen technique. There remains a great degree of heterogeneity in the patient population, and the surgical techniques described. The open abdomen technique has been used in both military and civilian trauma and vascular and general surgery emergencies. Given the lack of consistent practice, the Eastern Association for the Surgery of Trauma (EAST) Practice Management Guidelines Committee convened a study group to establish recommendations for the use of open abdomen techniques in both trauma and nontrauma surgery. This has been a major undertaking and has been divided into two parts. The EAST practice management guidelines for the open abdomen part 1 “Damage Control” have been published.[1]
During the development of the open abdomen part II “Management of the Open Abdomen,” the current literature remains contentious at best, current methods of treatment continue to change rapidly, and patient populations are so heterogeneous that clear recommendations could not be provided. What follows is a thorough review of the current literature for the management of the open abdomen: part 2 “Management of the Open Abdomen” and provides clinical direction regarding the following specific topics.
- Early and Delayed Abdominal Fascial Closure (DAFC).
- Management of intestinal fistula in the setting of the open abdomen.
- Management of the planned ventral hernia.
Process
A computerized search of the National Library of Medicine Medline database was undertaken using the PubMed Entrez interface. English language citations were identified during the period of 1984 through 2009 using the primary search strategies outlined. Given the complexity of this literature, several strategies were necessary to appropriately capture the breadth of evidence on the topic. The search excluded case reports, reviews, letters/commentary, editorials, and articles focusing only on pediatric participants.
The PubMed Related Articles algorithm was also used to identify additional articles similar to the items retrieved by the primary strategy, in addition to hand searching of the reference lists of key articles retrieved by the searches. Of ∼1,300 articles identified by these two techniques, only prospective or retrospective studies examining open abdominal management were selected, consisting of 79 institutional studies evaluating open abdomen management strategies in the adult surgical/critical care population. The articles were reviewed by a group of 16 surgeons who collaborated to produce this clinical review. The chair, vice chair, and three committee members (JJD, WD, MO) reviewed all the articles to categorize them into the three study topics. They were distributed to all members of the study group for critical review. Each committee member was to answer the following three questions of each article reviewed:
- What is the class of evidence in the article?
- Are the results of the article valid based on the data presented?
- What is your conclusion based on the evidence the article provides.
Review
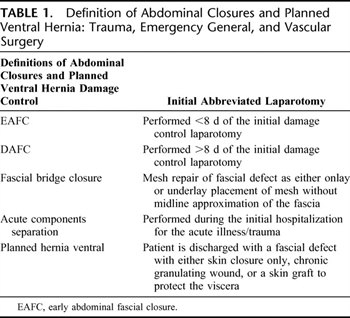
Table 1. Definition of Abdominal Closures and Planned Ventral Hernia: Trauma, Emergency General, and Vascular Surgery
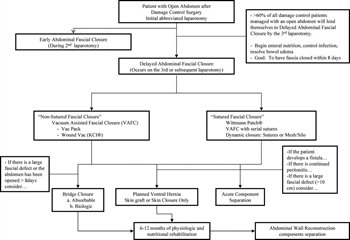
Figure 1. The closure of the open abdomen in trauma, emergency general, and vascular surgery flow diagram.
During the development of this review, a common language for the closure of the open abdomen was developed, which is provided in Table 1. Figure 1 is a proposed flow diagram for the closure of the open abdomen in trauma, emergency general, and vascular surgery.
Early Abdominal Fascial Closure
Timing
Trauma surgeons have gained an immense amount of experience with multiple techniques used to achieve abdominal closure of the open abdomen, but questions still remain. How long can the abdomen remain open? When does the risk of complications begin to increase? Is there a specific technique that is better than the rest for closing the open abdomen? At what point should all attempts at delayed fascial closure be abandoned and a planned ventral hernia performed? Miller et al.,[2] in a study of 344 damage control laparotomies demonstrated that early abdominal fascial closure can be achieved in the majority (63%) of damage control cases during the initial re-laparotomy. They showed that DAFC before 8 days was associated with fewer complications: 12% in those closed before 8 days and 52% closed after 8 days. Yet, in a study of trauma patients with an open abdomen, massive visceral edema, and loss of domain, fascial closure could be achieved using the V.A.C. therapy (vacuum-assisted closure, KCI, San Antonio, TX) overtime out to a 4-week period with acceptable complication rates.[3] With this degree of variation in timing to closure and the dreaded risk of life threatening complications more data were needed.
Delayed Abdominal Fascial Closure
Techniques (Nontraumatic/Traumatic Fascial Closure)

Table 2. Delayed Abdominal Fascial Closure

Table 2. Delayed Abdominal Fascial Closure (continued)
Multiple studies have shown that DAFC is safe and effective at achieving successful fascial closure in 65% to 100% of patients with an open abdomen.[2][4–25] There is evidence that vacuum-assisted closure devices (VACD) facilitate delayed primary fascial closure with high success rates and low morbidity.[3–5][7][13][14][16–18][26] The literature describes both commercially available devices (V.A.C. therapy) as well as “home make” noncommercial “vacuum packed–negative pressure dressing” devices as being helpful in achieving DAFC (Table 2).
In the setting of intra-abdominal sepsis, the effectiveness of VACD to achieve DAFC has not been as successful as the experience seen in trauma patients.[27] Wondberg et al.[28] studied 30 patients with intra-abdominal sepsis and an open abdomen. They showed that only 33% of the study group was able to achieve DAFC with the use of the V.A.C. therapy KCI. Failure to achieve DAFC is associated with significant financial cost, increased morbidity including wound infections and the formation of intestinal fistula.[29–31] Although, studies have shown that using VACD in conjunction with dynamic serial fascial advancement, can achieve fascial closure with success rates of 86% to 100% in trauma patients.[4][7][18][32]
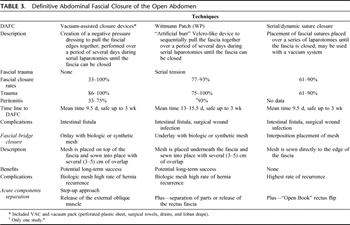
Table 3. Definitive Abdominal Fascial Closure of the Open Abdomen
The Wittmann Patch (Starsurgical, Burlington, WI), an “artificial burr” Velcro-like device that is sutured to the abdominal fascia, when used to manage an open abdomen has been shown to facilitates DAFC with a success rate >80% in a group of mixed trauma and abdominal sepsis patients.[10][23] The Wittmann Patch can be used as a successful tool to provide dynamic tension in a process toward fascial closure.[28][29] Similar to the Wittmann Patch, the use of temporary prosthetic mesh (most commonly polytetrafluoroethylene) with serial tightening/pleating has resulted in fascial closure rates from 89% to 100%.[14–21] Serial/dynamic suture tightening, a technique involving repeated partial closure of the fascia, has also been used to achieve DAFC at rates between 61% and 90%.[6][12][15]Table 3 describes the most commonly used abdominal closure surgical techniques and the differences between them.
There is one randomized prospective study comparing various techniques for DAFC. Bee et al.[33]compared the use of a VACD versus using a temporary polyglactin mesh and showed no difference in the rate of DAFC (31% vs. 26%). However, the success rates of DAFC in this study are significantly lower than other published studies, making the results difficult to interpret.
Fascial Bridge Closure
It has been previously described, a patient with an open abdomen can undergo multiple re-operations with progressive closure of the fascial defect, with or without the use of a VADC, and have their fascial defect closed.[3] In the setting of ongoing intra-abdominal infection or the formation of an enterocutaneous fistula abdominal fascial closure is often not possible.[19] Fascial closure may not be possible because of ongoing visceral edema with loss of abdominal domain or from loss of fascia from infection. At this point, a fascial bridge closure of the resulting abdominal fascial defect may be considered. The abdominal viscera will become cocoon in the 14-day period to 21-day period. Attempting re-enter into the abdomen cavity to free the visceral off the abdominal wall to allow for an easier abdominal fascial closure is both difficult and dangerous.
The surgeon is limited in the available surgical options: (1) bridge repair of the fascial defect using a mesh to create a bridge closure, (2) performing an acute abdominal wall reconstruction using most commonly a version of component separation, or (3) a planned ventral hernia.
Fansler et al.[34] reported their experience with the fascial bridge closure of the open abdomen with permanent prosthetic mesh. In a series of combined trauma and abdominal sepsis patients, polypropylene was used as a fascial bridge for early definitive closure. They had significant complications including a 50% enterocutaneous fistula rate, which were noted with the use of polypropylene mesh. Voyles et al.[35] reported a similar experience with a high rate of complications and fistula formation. The association of synthetic prosthetic mesh with bacterial colonization is well known. Once colonized or infected, the prosthetic mesh acts as a chronic source of contamination.[27][33][36] The use of permanent prosthetic mesh such as polypropylene, polytetrafluoroethylene, and polyester products has been abandoned in these circumstances because of the high rates of complications seen with their use.
Biological mesh material has been commercially available for almost 10 years. Biological mesh originates from human donors, bovine, and porcine animals. Biological mesh has been successfully used to bridge the defect as a result of an open abdomen. Human acellular dermal matrix (HADM) (AlloDerm, LifeCell Corp.) has been shown to be successfully used as a fascial bridge after open abdomen in multiple studies.[19][37–39] HADM does not seem to form significant adhesions, seems to tolerate bacterial contamination, and does not require removal in the setting of infection.[19][37][38][40–42]
Also, HADM has been successfully used for tissue coverage and closure of large traumatic wounds in the setting of significant skin and soft tissue loss.[32] Once the HADM has developed a good granulated tissue base, a skin graft can be placed. The authors noted that when no soft-tissue coverage is available, keeping the graft moist is critical to the graft's survival. Moist saline dressings or KCI V.A.C. therapy are most often used for this purpose. Bacterial colonization with overgrowth can occur on the grafts. This has been reported in the early postoperative phase and before the graft has had time to revascularize. The use of silver sulfadiazine or sulfamylon-soaked dressings on the graft should decrease bacterial counts until vascular in-growth has occurred and may prevent early graft loss from infection.
The long-term success of using HADM as a fascial bridge for hernia repair after an open abdomen technique is unclear. There are a number of studies suggesting that the long-term strength of the HADM decreases overtime. This multifactorial may be attributable to collagen re-modeling, mesh attenuation, or tissue growth resulting in a high rate of hernia formation.[43][44] However, HADM bridge ventral hernia repairs have been performed after trauma and many patients have had definitive repairs.[16] Singh et al.,[38] report on 10 liver transplant patients treated with an open abdomen and closed with an HADM fascial bridge. In short-term follow-up (10 months), there were no cases of herniation noted. Conversely, de Moya et al.,[45] demonstrated that patients treated with an HADM bridge repairs and that at 1-year follow-up had evidence of recurrent hernia or significant abdominal wall laxity.
The use of HADM as a fascial bridge under the circumstances of the unclosable abdomen after damage control is supported by the available literature. It protects the viscera from fistulization and may provide definitive abdominal wall strength. Yet, the long-term results in providing definitive fascial strength are not known.
Acute Component Separation
One option for closure of the open abdomen is an acute abdominal wall reconstruction using the component separation techniques. Ramirez et al.,[46] were the first to describe the component separation technique for reconstruction of large abdominal wall fascial defect without the use of prosthetic mesh. In its basic form, the technique is as follows: (1) the anterior abdominal wall skin flaps are developed and dissected out to the anterior superior iliac spine and the chest wall, (2) the aponeurosis of the external oblique muscle is divided lateral to the semilunar line on to the chest wall to the level of the xiphoid, (3) free up the external oblique, which will allow the rectus myofascial component to be mobilized medially, and (4) the midline is sutured together. The component separation has become the most commonly used surgical technique for closure of large “planned” ventral hernias with a skin graft during the elective reconstructive phase.[11][47][48] Its use for acute definitive closure in the setting of an open abdomen has not been well studied. Formal component separation is generally considered an “elective” reconstructive technique. Its use in the acute setting in the face of resolving intra-abdominal sepsis, visceral, and abdominal wall edema as a result of systemic inflammatory response syndrome and ongoing systemic sepsis is not advisable. Once a formal component separation has been performed, it is eliminated as an option for later abdominal wall reconstruction.
There are at least three versions of the component separation technique. The original description by Ramirez et al. is described above. Another surgical technique is the “separation of parts” by the Memphis group. There is also a “open book” technique, which in addition to the lateral release of the external oblique, the rectus fascia (either anterior or posterior) is flipped into the midline using the linea alba as the fulcrum to extend the midline. The rectus roll-over technique by itself has been studied in the setting of definitive closure after the open abdomen in both trauma and general surgery patients. The anterior rectus fascia is incised near its lateral border on both sides, medialized, and sewn in the midline. In a series of 29 patients, the technique was used successfully to close defects up to 15 cm.[30] In follow-up of 65 months, no recurrent abdominal wall hernias were noted, although mid-abdominal bulging was noted in 50% of patients.
Enteroatmospheric Fistula as a Complication of the Open Abdomen
During the initial damage control laparotomy, the open abdomen technique is used for rapid re-entry into the abdomen. DAFC can be commonly achieved once all the intra-abdominal injuries have been addressed. In the setting of intra-abdominal sepsis and/or pancreatitis, DAFC is not as successful.[49]It is well recognized that the longer the time period to fascial closure, the higher the complication rates especially intestinal fistulas.[50][51] In addition, the obese patient is at increased risk of having more complications after damage control laparotomy and longer time period to primary fascial closure.[50][52] Trauma patients who required a prolong period of an open abdomen as part of their damage control management have five times the fistula rate verses those patients who were closed during the initial trauma laparotomy. The enteroatmospheric intestinal fistula results in the setting of the open abdomen. The fistula can develop as a result of an anastomotic leak with exposed suture lines, traumatized bowel, and nontraumatized bowel, which has been exposed for a period of time. This is one of the most devastating complications of the open abdomen. The foremost risk factors are the inability to perform primary abdominal facial closure in a timely manner, and deep space infections, and intra-abdominal abscess.[19]
The use of polypropylene mesh for bridge repair of the open abdomen has been shown to have unacceptably high rates of fistula complications and is no longer recommended for definitive closure in the acute setting of open abdominal management.[34] Fistulae arising during early clinical management of open abdomens result in leakage of intraluminal contents over the unprotected surface of bowel. The patient with an enteroatmospheric fistula has extremely complicated critical care, open abdomen, and nutritional management issues. Inadequate fistula management will result in acute protein calorie malnutrition, electrolyte disturbances, and prolonged hospitalization.[53]
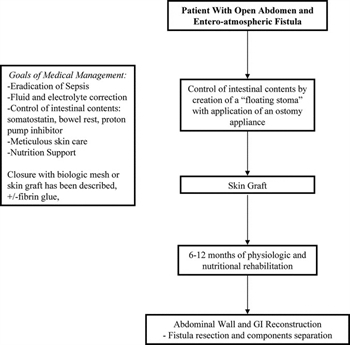
Figure 2. Intestinal fistula complicating the open abdomen flow diagram.
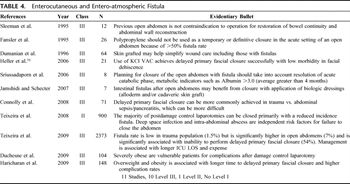
Table 4. Enterocutaneous and Entero-atmospheric Fistula
The key components of management of the patient with an enterocutaneous fistula are as follows: (1) sepsis control, (2) nutritional support, and (3) local wound care (Fig. 2). A key to treating entero-atmospheric fistulas is management of the initial inciting events and treatment of resulting complications. Source control and eradication of sepsis are essential. If possible, promote spontaneous closure and diminish the catabolic strain on the tissues.[54] In patients with intestinal fistulas with a tract or skin coverage, management of fistula output has been assisted by hormonal agents; however, randomized control trials do not favor octreotide as the standard of care.[55] Medical management has decreased the need for operative management of intestinal fistulas. More than 50% of patient with intestinal fistulas will require surgery for the control of sepsis and subsequent surgical repair for failure to close spontaneously.[56][57] Nutrition support either enteral or parenteral is considered a critical supportive measure to prevent malnutrition in an already debilitated patient.[58]Although, a full discussion of the management of intestinal fistula is beyond the scope of this article, Table 4 provides additional literature.
Local wound care can be extremely problematic in the patient with an open abdomen and an entero-cutaneus fistula. In an attempt to mitigate the inflammatory state preventing resolution of the entero-atmospheric fistula, Jamshidi and Schecter[59] treated seven patients with direct application of a biological dressing (HADM and/or cadaveric split thickness skin graft). Five of this series of seven closed with only two requiring further operative management. Physiologically similar, the application of skin graft to the granulated wound bed can have good results with as much as 93% graft take at 1 week.[60] The use of an innovative negative pressure dressings or the KCI V.A.C. therapy to collect the draining succus entericus to keep the open abdomen clean can be a daily wound management issue. An innovative option for improving wound care is the creation of the “floating stoma.” Recent case studies have described techniques for “floating stoma” with or without KCI V.A.C. therapy of the wound bed in attempts to simplify treatment before and after definitive repair.[61]
The restoration of gastrointestinal continuity at the time of the abdominal wall reconstruction is safe and the preferred treatment for entero-atmospheric fistula.[62] Success depends on the achievement of the goals set out in the management phase; the eradication of sepsis, optimizing nutrition status (albumin >3.25 g/dL), and delaying operative repair a minimum of 3 months to 12 months to allow for the development of a “neo-peritoneal cavity.”[63] An essential management priority is to stage the “elective” gastrointestinal reconstruction when the patient's sepsis has resolved. After the inflammatory process within the abdominal cavity has resolved, the intra-abdominal adhesions will progress through the various stages of inflammation to vascularize and loosen fibrous adhesions resulting in a safer operative procedure.[64] Even in the most optimized patient, entero-atmospheric fistulas remain among the most challenging problems, a surgeon will face.
Planned Ventral Hernia
Fabian et al.[65] and other authors are credited with the initial description of the stages of damage control. The goals of damage control are (1) patient survival, (2) reconstruction of the patient's traumatic injuries with the final goal and (3) being abdominal fascial closure.[47][62][66][67] As noted above, there are multiple techniques to achieve early or DAFC. When this is not possible, the planned ventral hernia technique is used.
Once it has been determined that the abdominal fascia will not come together because of massive visceral edema, loss of domain, and/or loss of abdominal wall tissue, the only option left is a planned ventral hernia or fascial bridge with biological mesh or absorbable mesh.[68][69] The initial goal of a planned ventral hernia is to keep the viscera within the abdominal cavity. This is accomplished by using absorbable mesh (Vicryl [Ethicon] or Dexon [Covidien]) to prevent evisceration. This allows time for the viscera to adhere together. This occurs during the course of 2 weeks to 3 weeks. Once the base of the open wound has granulated, a skin graft can be performed to cover the viscera. If the fascial defect is not large, another option is to elevate skin flaps and perform a skin only closure.[70]Caution must be exercised when elevating skin flaps in the setting of continued intra-abdominal sepsis, lack of source control, and massive visceral edema; because this setting has a high risk of skin flap infarction and flap loss. In this setting, allowing the wound base to progress to a good granulated base and proceeding to skin graft tissue coverage may be the safest option. Regardless of the technique used, visceral coverage is essential to decrease metabolic burden and prevent the formation of entero-atmospheric fistulae as a result of trauma from exposure or dressing changes.
Temporary abdominal closure with silicone sheets or Gortex has also been used to keep the abdominal contents from eviscerating.[11][71] This is done until the viscera have adhered together. The prosthetic mesh is removed and the granulation bed is skin grafted. Others have used bilateral bipedal flaps to cover the granulation bed with skin. The goal is to decrease the incidence of intestinal fistula formation.[37][72]
The introduction of biological mesh has been used in an attempt to do single stage repairs of ventral hernias.[16][38] The data to date suggests that the majority of patients repaired with biological mesh may develop laxity of the repair resulting in a hernia 6 months to 12 months later.[45] The role of biological mesh in the healing process has not been completely elucidated.
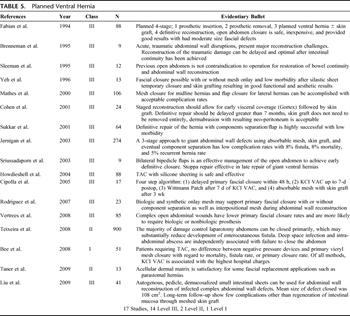
Table 5. Planned Ventral Hernia
The final stage of damage control is an “elective” abdominal wall reconstruction.[71][73] Because of the complexity of this topic, the EAST Open Abdomen Committee is in the process of developing a set of guidelines for abdominal wall reconstruction after the open abdomen. Questions regarding the preoperative evaluation, operative approach, and postoperative management and follow-up will be addressed (Table 5).
Conclusion
Damage control laparotomy in severe trauma, emergency general, and vascular surgery, in the setting of an abbreviated laparotomy as a result of physiologic exhaustion, has become the standard of care. The open abdomen technique has become an essential component of the procedure.
The management and closure of the open abdomen has developed into a separate surgical entity and remains a challenging problem to the surgeon. Several techniques have been developed to close the open abdomen. The majority of open abdomens can undergo early abdominal fascial closure during the initial re-laparotomy. If three or more laparotomies are required, DAFC can be achieved in the majority of cases using three surgical techniques (Wound Vac, Wittmann device, dynamic/suture closure with or without the use of a wound vacuum device). When the midline fascia cannot be approximated, two other techniques to consider are bridge closure with absorbable mesh or acute component separation.
The development of the entero-atmospheric fistula is a major clinical complication of the open abdomen. The development of the “floating stoma” and skin graft of the open abdomen becomes paramount in achieving control of enteric contents and wound sepsis. Finally, when DAFC cannot be achieved, one may proceed to plan ventral hernia, with the hope of accomplishing abdominal wall reconstruction in the future.
Future Direction

Table 6. Classification of the OA
The management of the open abdomen remains a very heterogeneous area of study. This is due to various issues such as the etiologies of the open abdomen (trauma, emergency general, and vascular surgery) and the presence of intra-abdominal sepsis. In addition, there are no accepted classification systems for the open abdomen. Recently, a consensus meeting of experts was held in January 2009 to propose a classification system for the open abdomen.[74] The classification is simple and can be applied to future studies. Currently, there is no sponsoring organization of the classification proposed and it has not been studied or validated. However, a standard classification system of the open abdomen is necessary, if a scientific approach is to be taken in regards to this vexing clinical problem (Table 6).
References
- Diaz JJ Jr, Cullinane DC, Dutton WD, et al. The management of the open abdomen in trauma and emergency general surgery: part 1-damage control. J Trauma.2010;68:1425–1438. View Full Text| PubMed | CrossRef
- Miller RS, Morris JA Jr, Diaz JJ Jr, Herring MB, May AK. Complications after 344 damage-control open celiotomies. J Trauma.2005;59:1365–1371. View Full Text| PubMed | CrossRef
- Miller PR, Thompson JT, Faler BJ, Meredith JW, Chang MC. Late fascial closure in lieu of ventral hernia: the next step in open abdomen management. J Trauma.2002;53:843–849. View Full Text| PubMed | CrossRef
- Cothren CC, Moore EE, Johnson JL, Moore JB, Burch JM. One hundred percent fascial approximation with sequential abdominal closure of the open abdomen. Am J Surg.2006;192:238–242. PubMed| CrossRef
- Defranzo AJ, Pitzer K, Molnar JA, et al. Vacuum-assisted closure for defects of the abdominal wall. Plast Reconstr Surg.2008;121:832–839. View Full Text| PubMed | CrossRef
- Gaddnas F, Saarnio J, Ala-Kokko T, Laurila J, Koivukangas V. Continuous retention suture for the management of open abdomen: a high rate of delayed fascial closure. Scand J Surg.2007;96:301–307. PubMed| CrossRef
- Garner GB, Ware DN, Cocanour CS, et al. Vacuum-assisted wound closure provides early fascial reapproximation in trauma patients with open abdomens. Am J Surg.2001;182:630–638. PubMed| CrossRef
- Gonullu D, Koksoy FN, Demiray O, Ozkan SG, Yucel T, Yucel O. Laparostomy in patients with severe secondary peritonitis. Ulus Travma Acil Cerrahi Derg.2009;15:52–57.
- Wittman DH. Staged abdominal repair: development and current practice of an advanced operative technique for defuse suppurative peritonitis. Acta Chir Austriaca.2000;32:171–178.
- Hadeed JG, Staman GW, Sariol HS, Kumar S, Ross SE. Delayed primary closure in damage control laparotomy: the value of the Wittmann patch. Am Surg.2007;73:10–12. PubMed
- Howdieshell TR, Proctor CD, Sternberg E, Cue JI, Mondy JS, Hawkins ML. Temporary abdominal closure followed by definitive abdominal wall reconstruction of the open abdomen. Am J Surg.2004;188:301–306. PubMed| CrossRef
- Koniaris LG, Hendrickson RJ, Drugas G, Abt P, Schoeniger LO. Dynamic retention: a technique for closure of the complex abdomen in critically ill patients. Arch Surg.2001;136:1359–1362. View Full Text| PubMed | CrossRef
- Perez D, Wildi S, Demartines N, Bramkamp M, Koehler C, Clavien PA. Prospective evaluation of vacuum-assisted closure in abdominal compartment syndrome and severe abdominal sepsis. J Am Coll Surg.2007;205:586–592. PubMed| CrossRef
- Petersson U, Acosta S, Bjorck M. Vacuum-assisted wound closure and mesh-mediated fascial traction—a novel technique for late closure of the open abdomen. World J Surg.2007;31:2133–2137. PubMed| CrossRef
- Reimer MW, Yelle JD, Reitsma B, Doumit G, Allen MA, Bell MS. Management of open abdominal wounds with a dynamic fascial closure system. Can J Surg.2008;51:209–214. View Full Text| PubMed
- Scott BG, Welsh FJ, Pham HQ, et al. Early aggressive closure of the open abdomen. J Trauma.2006;60:17–22. View Full Text| PubMed| CrossRef
- Subramonia S, Pankhurst S, Rowlands BJ, Lobo DN. Vacuum-assisted closure of postoperative abdominal wounds: a prospective study. World J Surg.2009;33:931–937. PubMed| CrossRef
- Suliburk JW, Ware DN, Balogh Z, et al. Vacuum-assisted wound closure achieves early fascial closure of open abdomens after severe trauma. J Trauma.2003;55:1155–1160. View Full Text| PubMed | CrossRef
- Teixeira PG, Salim A, Inaba K, et al. A prospective look at the current state of open abdomens. Am Surg.2008;74:891–897. PubMed
- Tieu BH, Cho SD, Luem N, Riha G, Mayberry J, Schreiber MA. The use of the Wittmann Patch facilitates a high rate of fascial closure in severely injured trauma patients and critically ill emergency surgery patients. J Trauma.2008;65:865–870. View Full Text| PubMed | CrossRef
- Vertrees A, Kellicut D, Ottman S, Peoples G, Shriver C. Early definitive abdominal closure using serial closure technique on injured soldiers returning from Afghanistan and Iraq. J Am Coll Surg.2006;202:762–772. PubMed| CrossRef
- Vertrees A, Greer L, Pickett C, et al. Modern management of complex open abdominal wounds of war: a 5-year experience. J Am Coll Surg.2008;207:801–809. PubMed| CrossRef
- Weinberg JA, George RL, Griffin RL, et al. Closing the open abdomen: improved success with Wittmann Patch staged abdominal closure. J Trauma.2008;65:345–348. View Full Text| PubMed | CrossRef
- Navsaria PH, Bunting M, Omoshoro-Jones J, Nicol AJ, Kahn D. Temporary closure of open abdominal wounds by the modified sandwich-vacuum pack technique. Br J Surg.2003;90:718–722. View Full Text| PubMed | CrossRef
- Stone PA, Hass SM, Flaherty SK, DeLuca JA, Lucente FC, Kusminsky RE. Vacuum-assisted fascial closure for patients with abdominal trauma. J Trauma.2004;57:1082–1086. View Full Text| PubMed | CrossRef
- Miller PR, Meredith JW, Johnson JC, Chang MC. Prospective evaluation of vacuum-assisted fascial closure after open abdomen: planned ventral hernia rate is substantially reduced. Ann Surg.2004;239:608–614. View Full Text| PubMed | CrossRef
- Tsuei BJ, Skinner JC, Bernard AC, Kearney PA, Boulanger BR. The open peritoneal cavity: etiology correlates with the likelihood of fascial closure. Am Surg.2004;70:652–656. PubMed
- Wondberg D, Larusson HJ, Metzger U, Platz A, Zingg U. Treatment of the open abdomen with the commercially available vacuum-assisted closure system in patients with abdominal sepsis: low primary closure rate. World J Surg.2008;32:2724–2729.PubMed| CrossRef
- Cipolla J, Stawicki SP, Hoff WS, et al. A proposed algorithm for managing the open abdomen. Am Surg.2005;71:202–207. PubMed
- Kushimoto S, Yamamoto Y, Aiboshi J, et al. Usefulness of the bilateral anterior rectus abdominis sheath turnover flap method for early fascial closure in patients requiring open abdominal management. World J Surg.2007;31:2–8. PubMed| CrossRef
- Vogel TR, Diaz JJ, Miller RS, et al. The open abdomen in trauma: do infectious complications affect primary abdominal closure? Surg Infect (Larchmt).2006;7:433–441. PubMed| CrossRef
- Awad SS, Rao RK, Berger DH, Albo D, Bellows CF. Microbiology of infected acellular dermal matrix (AlloDerm) in patients requiring complex abdominal closure after emergency surgery. Surg Infect (Larchmt).2009;10:79–84. PubMed| CrossRef
- Bee TK, Croce MA, Magnotti LJ, et al. Temporary abdominal closure techniques: a prospective randomized trial comparing polyglactin 910 mesh and vacuum-assisted closure. J Trauma.2008;65:337–342. View Full Text| PubMed | CrossRef
- Fansler RF, Taheri P, Cullinane C, Sabates B, Flint LM. Polypropylene mesh closure of the complicated abdominal wound. Am J Surg.1995;170:15–18. PubMed| CrossRef
- Voyles CR, Richardson JD, Bland KI, Tobin GR, Flint LM, Polk HC Jr. Emergency abdominal wall reconstruction with polypropylene mesh: short-term benefits versus long-term complications. Ann Surg.1981;194:219–223. View Full Text| PubMed| CrossRef
- Losanoff JE, Richman BW, Jones JW. Temporary abdominal coverage in critically ill patients. Arch Surg.2002;137:1078. View Full Text| PubMed| CrossRef
- Guy JS, Miller R, Morris JA Jr, Diaz J, May A. Early one-stage closure in patients with abdominal compartment syndrome: fascial replacement with human acellular dermis and bipedicle flaps. Am Surg.2003;69:1025–1028. PubMed
- Singh MK, Rocca JP, Rochon C, Facciuto ME, Sheiner PA, Rodriguez-Davalos MI. Open abdomen management with human acellular dermal matrix in liver transplant recipients. Transplant Proc.2008;40:3541–3544. PubMed| CrossRef
- Taner T, Cima RR, Larson DW, Dozois EJ, Pemberton JH, Wolff BG. The use of human acellular dermal matrix for parastomal hernia repair in patients with inflammatory bowel disease: a novel technique to repair fascial defects. Dis Colon Rectum.2009;52:349–354. View Full Text| PubMed | CrossRef
- Liu L, Li JS, Li N, Ren JA, Zhao YZ. Reconstruction of infected complex abdominal wall defects with autogenous pedicled demucosalized small intestinal sheet. Surgery.2009;145:114–119. View Full Text| PubMed | CrossRef
- Diaz JJ Jr, Conquest AM, Ferzoco SJ, et al. Multi-institutional experience using human acellular dermal matrix for ventral hernia repair in a compromised surgical field. Arch Surg.2009;144:209–215. PubMed| CrossRef
- Patton JH Jr, Berry S, Kralovich KA. Use of human acellular dermal matrix in complex and contaminated abdominal wall reconstructions. Am J Surg.2007;193:360–363. PubMed| CrossRef
- Bluebond-Langner R, Keifa ES, Mithani S, Bochicchio GV, Scalea T, Rodriguez ED. Recurrent abdominal laxity following interpositional human acellular dermal matrix. Ann Plast Surg.2008;60:76–80. View Full Text| PubMed | CrossRef
- Jin J, Rosen MJ, Blatnik J, et al. Use of acellular dermal matrix for complicated ventral hernia repair: does technique affect outcomes? J Am Coll Surg.2007;205:654–660. PubMed| CrossRef
- de Moya MA, Dunham M, Inaba K, et al. Long-term outcome of acellular dermal matrix when used for large traumatic open abdomen. J Trauma.2008;65:349–353. View Full Text| PubMed | CrossRef
- Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg.1990;86:519–526. View Full Text| PubMed | CrossRef
- Rodriguez ED, Bluebond-Langner R, Silverman RP, et al. Abdominal wall reconstruction following severe loss of domain: the R Adams Cowley Shock Trauma Center algorithm. Plast Reconstr Surg.2007;120:669–680. View Full Text| PubMed | CrossRef
- Vargo D. Component separation in the management of the difficult abdominal wall. Am J Surg.2004;188:633–637. PubMed| CrossRef
- Connolly PT, Teubner A, Lees NP, Anderson ID, Scott NA, Carlson GL. Outcome of reconstructive surgery for intestinal fistula in the open abdomen. Ann Surg.2008;247:440–444. View Full Text| PubMed | CrossRef
- Haricharan RN, Dooley AC, Weinberg JA, et al. Body mass index affects time to definitive closure after damage control surgery. J Trauma.2009;66:1683–1687. View Full Text| PubMed | CrossRef
- Teixeira PG, Inaba K, DuBose J, et al. Enterocutaneous fistula complicating trauma laparotomy: a major resource burden. Am Surg.2009;75:30–32. PubMed
- Duchesne JC, Schmieg RE Jr, Simmons JD, Islam T, McGinness CL, McSwain NE, Jr. Impact of obesity in damage control laparotomy patients. J Trauma.2009;67:108–112. View Full Text| PubMed | CrossRef
- Fischer PE, Fabian TC, Magnotti LJ, et al. A ten-year review of enterocutaneous fistulas after laparotomy for trauma. J Trauma.2009;67:924–928. View Full Text| PubMed | CrossRef
- Sancho JJ, di CJ, Nubiola P, et al. Randomized double-blind placebo-controlled trial of early octreotide in patients with postoperative enterocutaneous fistula. Br J Surg.1995;82:638–641.View Full Text| PubMed | CrossRef
- Evenson AR, Fischer JE. Current management of enterocutaneous fistula. J Gastrointest Surg.2006;10:455–464. PubMed| CrossRef
- Draus JM Jr, Huss SA, Harty NJ, Cheadle WG, Larson GM. Enterocutaneous fistula: are treatments improving? Surgery.2006;140:570–576. View Full Text| PubMed | CrossRef
- Hollender LF, Meyer C, Avet D, Zeyer B. Postoperative fistulas of the small intestine: therapeutic principles. World J Surg.1983;7:474–480. PubMed| CrossRef
- Dudrick SJ, Maharaj AR, McKelvey AA. Artificial nutritional support in patients with gastrointestinal fistulas. World J Surg.1999;23:570–576. PubMed| CrossRef
- Jamshidi R, Schecter WP. Biological dressings for the management of enteric fistulas in the open abdomen: a preliminary report. Arch Surg.2007;142:793–796. View Full Text| PubMed | CrossRef
- Dumanian GA, Llull R, Ramasastry SS, Greco RJ, Lotze MT, Edington H. Postoperative abdominal wall defects with enterocutaneous fistulae. Am J Surg.1996;172:332–334. PubMed| CrossRef
- Scaff DW, Brooks AJ, Bilski T, Gallagher J, Kauder D. A technique for management of the open abdomen in the presence of a fistula. Injury Extra.2007;38:43–48.
- Sleeman D, Sosa JL, Gonzalez A, et al. Reclosure of the open abdomen. J Am Coll Surg.1995;180:200–204. PubMed
- Sriussadaporn S, Sriussadaporn S, Kritayakirana K, Pak-art R. Operative management of small bowel fistulae associated with open abdomen. Asian J Surg.2006;29:1–7. PubMed| CrossRef
- Lynch AC, Delaney CP, Senagore AJ, Connor JT, Remzi FH, Fazio VW. Clinical outcome and factors predictive of recurrence after enterocutaneous fistula surgery. Ann Surg.2004;240:825–831. View Full Text| PubMed | CrossRef
- Fabian TC, Croce MA, Pritchard FE, et al. Planned ventral hernia. Staged management for acute abdominal wall defects. Ann Surg.1994;219:643–650. View Full Text| PubMed | CrossRef
- Yeh KA, Saltz R, Howdieshell TR. Abdominal wall reconstruction after temporary abdominal wall closure in trauma patients. South Med J.1996;89:497–502. View Full Text| PubMed | CrossRef
- Brenneman FD, Boulanger BR, Antonyshyn O. Surgical management of abdominal wall disruption after blunt trauma. J Trauma.1995;39:539–544. View Full Text| PubMed | CrossRef
- Jernigan TW, Fabian TC, Croce MA, et al. Staged management of giant abdominal wall defects: acute and long-term results. Ann Surg.2003;238:349–355. View Full Text| PubMed
- Smith PC, Tweddell JS, Bessey PQ. Alternative approaches to abdominal wound closure in severely injured patients with massive visceral edema. J Trauma.1992;32:16–20. View Full Text| PubMed | CrossRef
- Mathes SJ, Steinwald PM, Foster RD, Hoffman WY, Anthony JP. Complex abdominal wall reconstruction: a comparison of flap and mesh closure. Ann Surg.2000;232:586–596. View Full Text| PubMed | CrossRef
- Cohen M, Morales R Jr, Fildes J, Barrett J. Staged reconstruction after gunshot wounds to the abdomen. Plast Reconstr Surg.2001;108:83–92. View Full Text| PubMed | CrossRef
- Sriussadaporn S, Pak-art R, Bunjongsat S. Immediate closure of the open abdomen with bilateral bipedicle anterior abdominal skin flaps and subsequent retrorectus prosthetic mesh repair of the late giant ventral hernias. J Trauma.2003;54:1083–1089. View Full Text| PubMed | CrossRef
- Sukkar SM, Dumanian GA, Szczerba SM, Tellez MG. Challenging abdominal wall defects. Am J Surg.2001;181:115–121. PubMed| CrossRef
- Björck M, Bruhin A, Cheatham M, et al. Classification—important step to improve management of patients with an open abdomen. World J Surg.2009;33:1154–1157. PubMed| CrossRef
- Heller L, Levin SL, Butler CE. Management of abdominal wound dehiscence using vacuum assisted closure in patients with compromised healing. Am J Surg. 2006;191:165–172. PubMed| CrossRef