Open Abdomen Management, Review of abdominal wall reconstruction: Part 3
Published 2013
Citation: J Trauma. 75(3):376-386, September 2013
Authors
Diaz, Jose J. Jr. MD; Cullinane, Daniel C. MD; Khwaja, Kosar A. MD; Tyson, G. Hart MD; Ott, Mickey MD; Jerome, Rebecca MLIS, MPH; Kerwin, Andrew J. MD; Collier, Bryan R. DO; Pappas, Peter A. MD; Sangosanya, Ayodele T. MD; Como, John J. MD; Bokhari, Faran MD; Haut, Elliott R. MD; Smith, Lou M. MD; Winston, Eleanor S. MD; Bilaniuk, Jaroslaw W. MD; Talley, Cynthia L. MD; Silverman, Ronald MD; Croce, Martin A. MD
Author Information
From the University of Maryland School of Medicine (J.J.D.); John Hopkins Hospital (E.R.H.); and University of Maryland Medical Center (R.S.) Baltimore, Maryland; Marshfield Clinic (D.C.C.), Marshfield, Wisconsin; University of Texas Medical School at Houston (G.H.T.), Houston, Texas; Vanderbilt University Medical Center (M.O., R.J.), Nashville; University of Tennessee Knoxville (L.M.S.), Knoxville; University of Tennessee Medical Center (M.A.C.), Memphis, Tennessee; University of Florida (A.J.K.), Jacksonville; and Holmes Regional Medical Center (P.A.P.), Melbourne, Florida; Virginia Tech Carilion School of Medicine (B.R.C.), Roanoke, Virginia; School of Medicine and Dentistry (A.T.S.), University of Rochester Medical Center, Rochester, New York; MethoHealth Medical Center (J.J.C.), Cleveland, Ohio; Cook County Hospital (F.B.), Chicago, Illinois; Baystate Medical Center (E.S.W.), Springfield, Massachusetts; Morristown Memorial Hospital (J.W.B.), Morristown, New Jersey; University of Kentucky Medical Center (C.L.T.), Lexington, Kentucky; and McGill University Health Centre (K.A.K.), Montreal, Quebec, Canada.
Submitted: February 1, 2013, Revised: March 22, 2013, Accepted: March 25 2013, Published online: August 9, 2013.
This study was presented at the 24th Annual Scientific Assembly of the Eastern Association for the Surgery of Trauma, January 25–29, 2011, in Naples, Florida.
Address for reprints: Jose J. Diaz, Jr, MD, Department of Surgery, University of Maryland School of Medicine, 22 South Greene St, S4D07, Baltimore MD 21201; email: jdiaz@umm.edu.
Overview
The development of the “damage control” concept and the utility of the open abdomen (OA) technique in trauma, general, and vascular surgical emergencies have resulted in improved survival of the critically ill or injured patient.[1] The indications for damage-control surgery and the management of the OA have been published in the two previous publications from the Eastern Association for the Surgery of Trauma (EAST) Practice Management Guidelines Committee: Open Abdomen Parts I and II.[2][3] Every surgical procedure carries with it benefits and risks, which may not manifest themselves until later. For the patients recovering with an OA, the decision of when and how to close the abdomen can become quite complicated. This is especially true if there has been loss of abdominal domain or after an enteroatmospheric fistula has occurred.
In the acute setting, once intra-abdominal injuries have been addressed, visceral edema has subsided, and the degree of bacterial contamination has minimized, the abdomen should be closed. The etiology of the intra-abdominal pathology determines the ability to close the abdomen. The highest rates of abdominal closure are in trauma patients, followed by vascular emergencies. The lowest rates of abdominal closure are in emergency general surgery, with pancreatitis having the lowest rate of abdominal closure.[4] In 1994, Fabian et al.[5]described the planned ventral hernia as the initial stage in the management of the OA.
During the last 30 years, patients with catastrophic injury or illness have survived with the use of damage control and a planned ventral hernia. There has been intense interest in both addressing the shortcomings of the planned ventral hernia and how to electively repair these very complex of ventral hernias. Our aim was to develop an organized evidence-based approach to the management of the elective repair of the planned ventral hernia.
Patients and Methods
An extensive computerized literature search was performed using the National Library of Medicine, National Institutes of Health, MEDLINE database, www.guidelines.gov, and EMBASE. This was performed using PubMed Entrez interface www.pubmed.gov. An MLS (master of library and information science) librarian performed the search. Only English language articles were used. The focus was on the “elective” and “planned” repair of complex abdominal wall defects and abdominal wall reconstruction published between 1984 and 2012. Given the complexity of this literature, several strategies were necessary to appropriately capture the breadth of evidence on the topic. The search excluded case reports, reviews, letters/commentary, editorials, and articles focusing only on pediatric participants.
There were 278 articles identified by the initial search criteria; only prospective or retrospective studies examining “complex ventral hernia,” “open abdominal management,” “abdominal wall reconstruction,” “component separation,” “laparoscopic component separation,” “endoscopic component separation,” “Rives-Stoppa hernia repair,” and “elective” complex ventral hernia repair were selected.
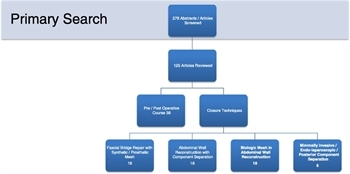
Figure 1. Flow diagram of articles identified and included in this review.
Four of the authors (J.J.D., G.H.T., M.O., and R.J.) reviewed all of the abstracts and selected 125 articles for full review by the study group. The articles were classified according to the EAST classes of evidence based on the 2000 EAST Primer for the development of practice management guidelines.[6] The reviewers were asked to provide their assessments of the articles based the on the EAST Primer and to formulate their conclusions based on the study results. All the reviewers’ comments were collated. and 99 articles were selected for the development of this review (Fig. 1).
The Review
Currently, there is no uniformly accepted classification or grading system for complex ventral hernias. Three classification systems have recently been proposed for complex ventral hernias. The leadership of the World Society of the Abdominal Compartment Syndrome has described a grading system for the acute OA.[7] The grading is as follows: Grade 1A, clean OA without adherence between bowel and abdominal wall or fixity of the abdominal wall (lateralization); Grade 1B, contamination OA without developing adherence/fixity; Grade 2A, clean OA developing adherence/fixity; Grade 2B, contaminated OA developing adherence/fixity; Grade 3, OA complicated by fistula formation; Grade 4, frozen OA with adherent/fixed bowel, unable to close surgically with or without fistula. The Ventral Hernia Working Group, which was supported by LifeCell Corporation, was specifically developed for late stage complex ventral hernia.[8] The grading system has four grades which are as follows: Grade 1, low risk (low risk of complications, no history of wound infections); Grade 2, comorbidities (smoker, obese, diabetic, immunosuppression, chronic obstructive pulmonary disease); Grade 3, potentially contaminated (previous wound infection, stoma, violation of the gastrointestinal tract); and Grade 4, infected (infected mesh, septic dehiscence). Neither grading system has been validated in clinical studies.
A recent study preoperatively classified different types of complex ventral hernias as follows: normal wound, healing (Type I), impaired wound healing (Type II), contaminated wound (Type III), massive weight loss (Type IV), and loss of domain (Type V). However, the study lacked the necessary power to delineate outcomes based on hernia types.[9]
Preoperative Considerations
Patients with significant comorbidities are at increased risk of adverse outcomes after ventral hernia repair.[10]Risk modification is an ideal approach to preoperative preparation for a major abdominal wall reconstruction. All patients should undergo cardiac and pulmonary preoperative evaluations. In the morbidly obese patient, identifying obstructive sleep apnea before surgery is helpful to modify the preoperative risk. Patients with a history of tobacco abuse must be counseled to stop smoking because they have the highest risk of wound complications, intestinal leak rates, and pulmonary complications.[11–15] Other risk factors associated with preoperative morbidity after ventral hernia repair failure are steroids,[16] chronic obstructive pulmonary disease, diabetes mellitus, body mass index (BMI) of greater than 30, previous wound infection, and infected mesh.[17]
A major decision point is the timing of abdominal wall reconstruction. In the setting of trauma or emergency surgery, the patient has survived a catastrophic illness or injury with an OA. Tissue coverage is generally achieved with either a skin closure only or a skin graft. A sufficient period must be allowed for healing to occur. Specifically, the abdominal adhesions must mature to the point where the viscera can be safely dissected free and the skin graft can be removed. While most abdominal reconstructive surgeons will recommend waiting a minimum of 6 months before a planned abdominal wall reconstruction, only one study has demonstrated this period as a safe approach without significantly affecting the morbidity rates.[18]
The patient with an enteroatmospheric fistula requires an organized approach to preoperative preparations and planning for fistula takedown with an abdominal wall reconstruction. The initial step is skin coverage during the early stages of the OA. With distal intestinal fistulas (especially colocutaneouas fistulas), patients may be fed enterally to allow for the benefits of enteral nutrition. Rarely, a very proximal enteroatmospheric intestinal fistula may be accessed with a feeding tube directly to feed the gut distally, once it has been demonstrated that a significant length of small bowel is present distal to the fistula. Aggressive parental nutritional support is often the only option to maintain the patient’s nutritional status. Wound healing vitamins (vitamin C) and antioxidants (Zn, selenium) have been used in this severely malnourished patient population.[19][20] Most of these recommendations arise from retrospective case reviews, case reports, or expert opinion.
Radiographic assessment of the abdominal wall anatomy before elective abdominal wall reconstruction is an essential step. Multidetector computer tomography can demonstrate the size of the ventral defect and assess the degree of abdominal loss of domain. Multidetector computer tomography has the ability to demonstrate the amount of tissue loss after trauma or necrotizing wound infections as well as the presence of heterotopic ossification within the scar can be assessed.[21]
Closure Techniques
Fascial Bridge and/or Repair With Synthetic/Prosthetic Mesh
The landmark study of 2000 Luijendijk et al.[22] demonstrated that large (>4 cm) repaired with mesh had a lower incidence of hernia recurrence. Since before this study, tension-free repair of large ventral hernias with prosthetic mesh (polypropylene or polytetrafluoroethylene [PTFE]) was the standard with hernia recurrence rates of 19% to 32% and complication rates of 10% to 17%.[23–25] The position of the mesh has a direct impact on the hernia recurrence rate, with underlay having a lower recurrence rates than onlay placement.[26] The majority of these cases were elective and not after trauma or abdominal catastrophes. They rarely had an intestinal fistula or an ostomy repaired at the same setting. Even without contamination, the risk of mesh infection was reported to be 10% to 17%.[27–32]
The mesh can be placed in the various positions and each has its benefits and risk:
- Onlay—placed directly on top of the anterior rectus fascia
- Inlay—interposition sewn directly to the edge of the fascia as a bridge repair for patients in whom the fascia cannot be directly reapproximated
- Sublay—positioned posterior to the rectus muscle between the muscle and the posterior rectus fascia, in the retrorectus space, just superficial to the peritoneum
- Underlay—positioned intra-abdominally, posterior to the rectus fascia and directly on the peritoneum
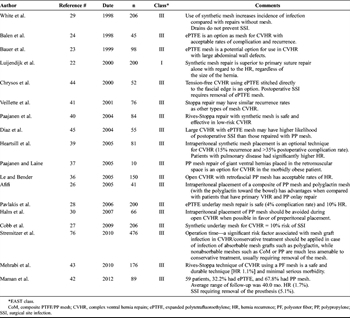
Table 1. Synthetic Ventral Hernia Repairs (Bridge and Modified “Rives-Stoppa”)
The “inlay” or “interpositional” placement of the mesh is not recommended because it is associated with the highest rate of recurrence followed by an “onlay” bridge repair technique.[33] There is no definitive evidence that “underlay/sublay” compared with “onlay” position of the mesh is superior. Each is equally effective for open surgical procedures for ventral hernia repairs.[34][35] The “sublay” position (posterior to the rectus muscle) allows for better tissue incorporation and lower chance of bacterial contamination when implanted adjacent to the rectus muscle. In addition, posterior rectus sheath and the peritoneum keep the mesh from coming into direct contact with the viscera.[36][37] The “sublay” in the “preperitoneal space or retrorectus fascial position” protects the mesh from direct contact with the viscera but requires an identifiable peritoneal layer to be dissected out and closed posterior to the mesh. Hernia recurrent rates of 3% have been reported, and short-term follow-up studies have shown a 2% to 15% recurrence rate.[12][38] When a sublay repair includes closure of the posterior fascia in the midline, the procedure is described as a “modified Rives-Stoppa repair.” Synthetic mesh is safe and effective technique in low-risk ventral hernia repairs with hernia recurrence rates of 1.1% to 18% and 6.8% infection rate.[39–41] The “underlay” position is the placement of the mesh directly on the peritoneum and is the default position (Table 1).
If a prosthetic mesh develops an infection, explantation is almost universally required, which will often result in an immediate hernia recurrence.[41][42] It is well documented that once PTFE has become infected, the only viable option is removal of the mesh.[43][44] PTFE should not be used for abdominal wall reconstruction after patients are managed with an OA. Prosthetic synthetic mesh remains a viable option in the repair of large ventral hernias but should be strictly limited to clean cases.
Abdominal Wall Reconstruction with Component Separation
Component separation was initially described by Ramirez et al.[45] as a tissue-only repair. The initial description of the component separation involved the development of large skin flaps off the anterior rectus fascia. The dissection exposed the aponeurosis of the external oblique fascia. The aponeurosis is divided longitudinally starting at the anterior superior iliac spine and onto the costal margin. In the majority of cases, the component separation technique will close an abdominal wall fascial defect of 15 cm to 20 cm in the midabdomen. There are other variations on the “component separation.” Fabian et al.[5] described what is commonly called the “separation of parts.” The anterior rectus fascia and muscle are separated from the posterior rectus fascia. The anterior rectus fascia and muscle are mobilized medially, allowing for the recreation of the linea alba. The lateral edge of the anterior rectus fascia is sewn to the medial edge of the posterior rectus fascia. This technique does result in three suture lines. Other described the release of the anterior rectus fascia in an anterior or posterior position.
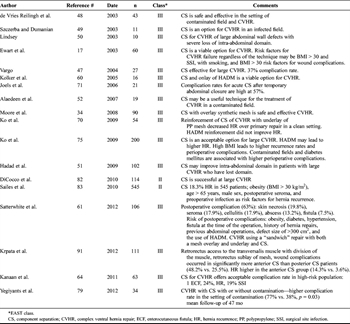
Table 2. Abdominal Wall Reconstruction With Component Separation
The initial reports of component separation repairs had significant morbidity with 37% to 39% wound complications and 32% hernia recurrence with follow-up period of 15 months.[46–50] Since component separation is a tissue-only repair, the technique was commonly applied to wounds with bacterial colonization or contaminated surgical fields.[51] Large skin flaps used to expose the external oblique muscles and subsequent dead space allow for the development of seromas and wound infections (Table 2).
Component Separation With Synthetic Mesh
Several studies have reported the use of prosthetic mesh to support the component separation repair in either an onlay and/or underlay position with the goal to decrease the hernia recurrence rates. The procedure still has significant morbidity, with wound infection rates of 10% to 35% and with a recurrence rate of 5.5% to 15% during a 50-month follow-up period.[34][38]
Bridge Repair With Biologic Mesh
Biologic mesh has been used extensively as an option to repair hernia defects in wounds with bacterial colonization or contamination. The hope was that a biologic mesh would be able to “resist” infection if the wound became infected. The theory was that a biologic mesh would quickly become vascularized and incorporated, allowing it to tolerate bacterial contamination better. A vascularized mesh would be able to bring inflammatory cells and antimicrobials to the wound. In the acute setting, the initial results were promising. The need for explantation of biologic mesh was low when used to close ventral defects, but many of these studies suffered from short follow-up times.
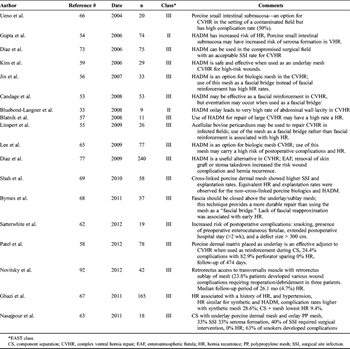
Table 3. Biologic Mesh in Abdominal Wall Reconstruction
Subsequent studies with longer follow-up times showed that most patients repaired with a biologic mesh positioned as a bridge repair developed an eventuation or attenuation of the repair described as hernia recurrence.[33][52–56] The most studied biologic mesh is human acellular dermal matrix (HADM) (LifeCell, Branchburg, NJ). There have been multiple theories why HADM had high failure rates: from the high content of elastin found in the dermal matrix, the remodeling/reabsorption of the implant over time, the constant increased intra-abdominal pressure resulting in attenuation, and the thinning out of the implant in the setting of the exposed wound. The common use of a negative-pressure dressing on the HADM might have also resulted in the thinning of the implant. The end result was increased propensity for weakness of the repair, resulting in recurrent ventral hernia. At this point, the use of a biologic mesh bridge repair without primary fascial closure is discouraged (Table 3).
Component Separation With Biologic Mesh Support
The next step in the evolution of abdominal wall reconstruction was to combine component separation with the biologic mesh reinforcement. Many surgeons began to combine complex gastrointestinal procedures, stoma repairs, and intestinal fistula repairs with abdominal wall reconstruction.
The position of the biologic mesh in the conjunction with component separation technique is felt to be critical to avoid recurrence. The sublay or underlay positions have been described, and both techniques seem to have similar hernia recurrence rates.[57][58] One further technical issue in placing the mesh in a “sublay” or “underlay” position is the suturing technique used to secure the mesh. The mesh should be sewn in using interrupted transfascial “U” sutures through the anterior abdominal wall.[55][58] The interrupted “U” suture technique allows for secure placement of the mesh against the abdominal wall and limits the degree of potential ischemia or edema to the tissues medial to the placement of the sutures. A continuous suture technique to secure the mesh should be avoided. The key step is the approximation of the linea albae/midline fascia, reestablishing the normal anatomy to the abdominal wall. In independent studies, Kolker et al.[59] and Satterwhite et al.[60][61] both have described a “sandwich” techniques using HADM as a dual layer with both an underlay and onlay with midline fascial approximation. Others have described using a biologic (porcine dermis) mesh underlay with a polypropylene mesh onlay in conjunction with a component separation and a 33% wound infection rate.[62]During the early experience of biologic mesh and component separation repairs in complex ventral hernia repairs, HADM was commonly used and an overall complication rate is as high as 19% to 50%.[63–66]
Porcine biologic non–cross-linked mesh has been used to support the repair of the component separation. Placing the mesh in an underlay position has been reported to have the best short-term outcomes with hernia recurrence rates of 7%.[67] One study compared 58 patients with cross-linked, non–cross-length porcine mesh and HADM. Cross-linked porcine mesh had relatively higher infection and explantation rates. Equivalent hernia recurrence and explantation rates were observed for the non–cross-linked porcine biologics and HADM. These data indicate that there is currently no ideal biologic for complex ventral hernia repair.[68]
One study compared polypropylene mesh with cadaveric dermis in an underlay position to support the component separation repair. Polypropylene mesh versus cadaveric mesh with a short-term follow-up had hernia recurrence rates of 11% versus 45%.[69]
Complications of Component Separation
The overall complication rate of abdominal wall reconstruction is in the range of 50% to 60%.[70] Intestinal fistula, hernia defect size of greater than 300 cm[2], active abdominal infection, and open repair have all been shown to be independent risk factors for 30-day readmission after complex ventral hernia repair.[71] Complications from abdominal wall reconstruction with component separation are of three major categories as follows: (1) wound infection, (2) flap loss, and (3) hernia recurrence.
First, the classical open approach to component separation requires the creation of large skin flaps to expose the aponeurosis of the external oblique fascia. This predisposes the wound to infection by the formation of seromas, along with possible skin and subcutaneous ischemia. After an abdominal catastrophe, the surgical field may remain colonized for a prolonged period and can increase the risk of infection.[7] With the increasing incidence of the complex ventral hernia, the surgeon has had a need for a product to help close abdominal wall defects in contaminated fields. This resulted in the rapid increase in the use of biologic mesh for repair of the complex ventral hernia in the compromised surgical fields.[72] Untreated postoperative complications have been associated with delayed presentation of prosthetic mesh infections most commonly with Staphylococcusspecies.[73] The rate of wound infection after component separation ranges from 24% to 33%.[74] Operative times greater than 4 hours have been shown to be a risk factor for the development wound infection.[75] Treatment of wound infections after a component separation requires a graduated approach. Wound infection is a spectrum of disease. Early wound infections are manifested as simple cellulitis and may be successfully treated medically with antibiotics alone. Late or deep-seated wound infections may require percutaneous or surgical drainage of infected subcutaneous collections.[72][76]
The second and more serious complication with component separation is flap loss cause by ischemia/necrosis. Anterior abdominal wall skin flap loss can be a catastrophic postoperative complication. Ischemia can range from a limited periincisional skin and fat necrosis to complete skin and soft tissue loss of large portions of the anterior abdominal wall. Precise surgical technique is an essential component to the success of skin flap survival. The plane of dissection must be directly along the anterior rectus fascia in the adventitial layer between the fascia and the subcutaneous tissue. This allows the superficial and deep capillary networks in the subcutaneous tissue to remain intact because they become the primary blood supply to these large skin flaps. In most series, the wound infection rate is reported to be 30%, and the skin flap necrosis rate is reported to 1%.[77] One study evaluated component separation for complex ventral hernia repairs and demonstrated a higher rate of complications in patients with contaminated wounds (77%) compared with clean wounds (38%).[78]
A modified periumbilical sparing technique that preserves the large arterial perforators from the periumbilical area has been shown to decrease skin flap loss.[79] Presence of old subcostal or transverse incisions is a risk factor for compromised circulation to the skin and subcutaneous tissue. However, a recent study from MD Anderson examined the use of component separation with previous incisional scars and stomas and showed no increase in tissue loss with an experienced reconstructive surgeon.[80]
The third major complication of component separation is hernia recurrence. In three recent series, the rate of hernia recurrence ranged from 5% to 14% to 30%.[74][78][81] In another recent study on 545 component separations, there was an 18.3% hernia recurrence rate. Obesity (BMI > 30 kg/m[2]), older than 65 years, male sex, postoperative seroma, and preoperative infection were identified as risk factors for hernia recurrence.[82]Moreover, the history of hernia repairs increases the risk of hernia recurrence.[83]
Minimally Invasive/Endolaparoscopic Anterior Component Separation/Posterior Component Separation

Table 4. Minimally Invasive/Endolaparoscopic/Posterior Component Separation
The most common morbidity of the component separation technique is wound complication rate. To help minimize these issues, several authors began to develop minimally invasive techniques to component separation. A key goal was to limit the dead space and preserve the feeding perforating vessels to the anterior skin. This newer surgical techniques has been shown to decrease the wound complications rates from 20% to 2%.[79] Both laparoscopic and minimally invasive surgical techniques for component separation have been described. A transverse incision is made medial to the anterior superior iliac spine and lateral to the rectus muscle, which allows one to dissect down to the external oblique fascia. The endo/laparoscopic technique use a hernia balloon to develop a plane in between the external and internal oblique muscles. The area is insufflated, and the external oblique aponeurosis can be seen anteriorly and divided.[84–87] In the minimally invasive technique, the external oblique aponeurosis is directly visualized and divided[88][89] while using a narrow Deaver-type retractor to elevate the tunnel. Both techniques allow the rectus muscle and fascial component to be mobilized medially and avoid creation of large dead space. These approaches preserve the rectus vascular perforators, which decrease the risk of wound infection and potential flap loss compared with the conventional approach (Table 4).
A retrorectus approach to the transversalis muscle with division of the muscle has been described.[90][91] In a study of 111 patients, they demonstrated a lower wound complication rate (48.2% vs. 25.5%) as well as a lower hernia recurrence rate (14.3% vs. 3.6%) versus the anterior component separation approach. A retrorectus sublay mesh is placed to support the repair similar to a Rives-Stoppa–type repair. This approach also eliminates creation of large skin flaps.
Perioperative/Postoperative Management
There are several other aspects of the perioperative abdominal wall reconstruction patient, which are essential to its success by preventing bacterial contamination. Prophylactic antibiotics should be dosed at least 2 hours before incision and every 4 hours.[92]
Intraoperatively, the patient’s core temperature should remain greater than 35°C during the entire case.[93]During the time of abdominal closure, pulmonary peak airway pressures should be monitored because the risk of developing intra-abdominal hypertension increases particular if there was loss of domain. With increasing plateau pressures of 9 cm H2O or greater, consideration must be given to keeping the patient intubated postoperatively to manage intra-abdominal hypertension and potential respiratory complications.[13] One study used preoperative assessment of loss of domain called component separation index. Computed tomographic scan was used to determine the angle of diastasis to predict the need for interposition mesh placement in addition to component separation.[94] Another study used intraoperative tensiometry as a decision tool for abdominal wall reconstruction. If a result exceeded 1.5 kp, component separation of the lateral abdominal wall was performed.[95] One study prospectively measured bladder pressures during abdominal wall reconstitution with component separation. They noted a bladder pressure of greater than 20 mm Hg correlated with an increase in postoperative complications.[96] Postoperative monitoring of bladder pressures should be performed to monitor for intra-abdominal hypertension.[97][98]
Conclusion
The OA technique has been used in traumatic, general surgical, and vascular surgical emergencies with great success. It is a heroic approach, which is work intensive at the front end. If the patient survives with a planned ventral hernia, there must be a focused approach to the planning of the abdominal wall reconstruction. There are multiple pitfalls during the preoperative, operative, and postoperative course. Knowledge of all aspects of this complex approach is essential if one is to successfully repair these complex ventral hernias. A void in the literature persists regarding the postoperative management of these patients, and future study should focus on these aspects of the surgical care.
Authorship
J.J.D., G.H.T., and M.O. contributed in the study design, initial literature search, and initial review and paring of the articles. J.J.D., G.H.T., M.O., and R.J. performed the initial screening of all abstracts and identification of articles to be review. J.J.D., D.C.C., K.A.K., G.H.T., M.O., R.J., A.J.K., B.R.C., P.A.P., A.T.S., J.C.J., F.B., E.R.H., L.M.S., E.S.W., J.W.B., C.L.T., R.S., and M.A.C. contributed in the EAST Primer Classification—2000 review, ranking, and written assessment of assigned articles. G.H.T., M.O., and J.J.D. contributed in the gathering, collating of the responses in categories, and database development. G.H.T., M.O., and J.J.D. contributed in the development and presentation of the project at the EAST plenary session 2011. G.H.T., M.O., and J.J.D. contributed in the gathering and incorporation of the recommendations from the EAST membership during the PMG plenary presentation. J.J.D., D.C.C., and K.A.K. were responsible for the manuscript development, writing, and additional literature search. J.J.D., J.C.J., and E.R.H. edited the manuscript.
Disclosure
The authors declare no conflicts of interest.
References
- Sutton E, Bochicchio GV, Bochicchio K, et al. Long term impact of damage control surgery: a preliminary prospective study. J Trauma. 2006; 61: 831–834; discussion 835–836.
- Diaz JJ Jr, Cullinane DC, Dutton WD, Jerome R, Bagdonas R, Bilaniuk JW, Collier BR, Como JJ, Cumming J, Griffen M, et al. The management of the open abdomen in trauma and emergency general surgery: part 1—damage control. J Trauma. 2010; 68: 1425–1438. View Full Text| PubMed| CrossRef
- Diaz JJ Jr, Dutton WD, Ott MM, Cullinane DC, Alouidor R, Armen SB, Bilanuik JW, Collier BR, Gunter OL, Jawa R, et al. Eastern Association for the Surgery of Trauma: a review of the management of the open abdomen—part 2 “Management of the open abdomen”. J Trauma. 2011; 71: 502–512. View Full Text| PubMed| CrossRef
- Tsuei BJ, Skinner JC, Bernard AC, Kearney PA, Boulanger BR. The open peritoneal cavity: etiology correlates with the likelihood of fascial closure. Am Surg. 2004; 70: 652–656. PubMed
- Fabian TC, Croce MA, Pritchard FE, Minard G, Hickerson WL, Howell RL, Schurr MJ, Kudsk KA. Planned ventral hernia. Staged management for acute abdominal wall defects. Ann Surg. 1994; 219: 643–650; discussion 651–653.
- Eastern Association for the Surgery of Trauma (EAST) Ad Hoc Committee on Practice Management Guideline Development 2000, Utilizing Evidence Based Outcome Measures to Develop Practice Management Guidelines: A Primer Available at: www.east.org/content/documents/east_pmg_primer.pdf. Accessed November 13, 2010.
- Björck M, Bruhin A, Cheatham M, Hinck D, Kaplan M, Manca G, Wild T, Windsor A., Batacchi S, Matano S, et al. Classification—important step to improve management of patients with an open abdomen. World J Surg. 2009; 33: 1154–1157. Crit Care. 2009;13:R194.
- Ventral Hernia Working Group, Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF, Rosen M, Silverman RP, Vargo D. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010; 148: 544–558. View Full Text| PubMed| CrossRef
- Hadeed JG, Walsh MD, Pappas TN, Pestana IA, Tyler DS, Levinson H, Mantyh C, Jacobs DO, Lagoo-Deenadalayan SA, Erdmann D. Complex abdominal wall hernias—a new classification system and approach to management based on review of 133 consecutive patients. Ann Plast Surg. 2011; 66: 497–503. View Full Text| PubMed| CrossRef
- Albright EL, Davenport DL, Roth JS. Preoperative functional health status impacts outcomes after ventral hernia repair. Am Surg. 2012; 78: 230–234. PubMed
- Dunne JR, Malone DL, Tracy JK, Napolitano LM. Abdominal wall hernias: risk factors for infection and resource utilization. J Surg Res. 2003; 111: 78–84. PubMed| CrossRef
- Novitsky YW, Porter JR, Rucho ZC, et al. Open preperitoneal retrofascial mesh repair for multiply recurrent ventral incisional hernias. J Am Coll Surg. 2006; 203: 283–289. PubMed| CrossRef
- Blatnik JA, Krpata DM, Pesa NL, Will P, Harth KC, Novitsky YW, Rowbottom JR, Rosen MJ. Predicting severe postoperative respiratory complications following abdominal wall reconstruction. Plast Reconstr Surg. 2012; 130: 836–841. View Full Text| PubMed| CrossRef
- Sørensen LT, Jørgensen T, Kirkeby LT, Skovdal J, Vennits B, Wille-Jørgensen P. Smoking and alcohol abuse are major risk factors for anastomotic leakage in colorectal surgery. Br J Surg. 1999; 86: 927–931. View Full Text| PubMed| CrossRef
- Kruschewski M, Rieger H, Pohlen U, Hotz HG, Buhr HJ. Risk factors for clinical anastomotic leakage and postoperative mortality in elective surgery for rectal cancer. Int J Colorectal Dis. 2007; 22: 919–927. PubMed| CrossRef
- Finan KR, Vick CC, Kiefe CI, Neumayer L, Hawn MT. Predictors of wound infection in ventral hernia repair. Am J Surg. 2005; 190: 676–681. PubMed| CrossRef
- Ewart CJ, Lankford AB, Gamboa MG. Successful closure of abdominal wall hernias using the components separation technique. Ann Plast Surg. 2003; 50: 269–273; discussion 273–264.
- Ekeh AP, McCarthy MC, Woods RJ, Walusimbi M, Saxe JM, Patterson LA. Delayed closure of ventral abdominal hernias after severe trauma. Am J Surg. 2006; 191: 391–395. PubMed| CrossRef
- Blass SC, Goost H, Tolba RH, Stoffel-Wagner B, Kabir K, Burger C, Stehle P, Ellinger S. Time to wound closure in trauma patients with disorders in wound healing is shortened by supplements containing antioxidant micronutrients and glutamine: a PRCT. Clin Nutr. 2012; 31: 469–467. PubMed| CrossRef
- Junge K, Rosch R, Anurov M, Titkova S, Ottinger A, Klinge U, Schumpelick V. Modification of collagen formation using supplemented mesh materials. Hernia. 2006; 10: 492–497. PubMed| CrossRef
- Aguirre DA, Santosa AC, Casola G, Sirlin CB. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at multi-detector row CT. Radiographics. 2005; 25: 1501–1520. PubMed| CrossRef
- Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, IJzermans JN, Boelhouwer RU, de Vries BC, Salu MK, Wereldsma JC, et al.A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med. 2000; 343: 392–398. View Full Text| PubMed| CrossRef
- Bauer JJ, Harris MT, Kreel I, Gelernt IM. Twelve-year experience with expanded polytetrafluoroethylene in the repair of abdominal wall defects. Mt Sinai J Med. 1999; 66: 20–25. PubMed
- Balen EM, Diez-Caballero A, Hernandez-Lizoain JL, et al. Repair of ventral hernias with expanded polytetrafluoroethylene patch. Br J Surg1998; 85: 1415–1418. View Full Text| PubMed | CrossRef
- Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004; 240: 578–583; discussion 583–585.
- Afifi RY. A prospective study between two different techniques for the repair of a large recurrent ventral hernia: a double mesh intraperitoneal repair versus onlay mesh repair. Hernia. 2005; 9: 310–315. PubMed| CrossRef
- Cobb WS, Carbonell AM, Kalbaugh CL, Jones Y, Lokey JS. Infection risk of open placement of intraperitoneal composite mesh. Am Surg. 2009; 75: 762–767; discussion 767–768.
- Pavlakis E, Avgerinos E, Filippou D, Pikoulis E, Tsatsoulis P, Skandalakis P. Open reconstruction of sizeable ventral hernias in the laparoscopic era. Am Surg. 2006; 72: 139–144. PubMed
- White TJ, Santos MC, Thompson JS. Factors affecting wound complications in repair of ventral hernias. Am Surg. 1998; 64: 276–280. PubMed
- Halm JA, de Wall LL, Steyerberg EW, Jeekel J, Lange JF. Intraperitoneal polypropylene mesh hernia repair complicates subsequent abdominal surgery. World J Surg. 2007; 31: 423–429; discussion 430.
- Collage RD, Rosengart MR. Abdominal wall infections with in situ mesh. Surg Infect (Larchmt). 2010; 11: 311–318. PubMed| CrossRef
- Choi JJ, Palaniappa NC, Dallas KB, Rudich TB, Colon MJ, Divino CM. Use of mesh during ventral hernia repair in clean-contaminated and contaminated cases: outcomes of 33,832 cases. Ann Surg. 2012; 255: 176–180. PubMed| CrossRef
- Bluebond-Langner R, Keifa ES, Mithani S, Bochicchio GV, Scalea T, Rodriguez ED. Recurrent abdominal laxity following interpositional human acellular dermal matrix. Ann Plast Surg. 2008; 60: 76–80. View Full Text| PubMed| CrossRef
- Moore M, Bax T, MacFarlane M, McNevin MS. Outcomes of the fascial component separation technique with synthetic mesh reinforcement for repair of complex ventral incisional hernias in the morbidly obese. Am J Surg. 2008; 195: 575–579; discussion 579.
- den Hartog D, Dur AH, Tuinebreijer WE, Kreis RW. Open surgical procedures for incisional hernias. Cochrane Database Syst Rev. 2008; 16: CD006438. PubMed
- Le H, Bender JS. Retrofascial mesh repair of ventral incisional hernias. Am J Surg. 2005; 189: 373–375. PubMed| CrossRef
- Paajanen H, Laine H. Operative treatment of massive ventral hernia using polypropylene mesh: a challenge for surgeon and anesthesiologist. Hernia. 2005; 9: 62–67. PubMed| CrossRef
- Heartsill L, Richards ML, Arfai N, Lee A, Bingener-Casey J, Schwesinger WH, Sirinek KR. Open Rives-Stoppa ventral hernia repair made simple and successful but not for everyone. Hernia. 2005; 9: 162–166. PubMed| CrossRef
- Paajanen H, Hermunen H. Long-term pain and recurrence after repair of ventral incisional hernias by open mesh: clinical and MRI study. Langenbecks Arch Surg. 2004; 389: 366–370. PubMed| CrossRef
- Veillette G, MacGillivray D, Whalen G. Practical experience with the Stoppa repair of ventral/incisional hernias. Conn Med. 2001; 65: 67–70. PubMed
- Maman D, Greenwald D, Kreniske J, Royston A, Powers S, Bauer J. Modified Rives-Stoppa technique for repair of complex incisional hernias in 59 patients. Ann Plast Surg. 2012; 68: 190–193. View Full Text| PubMed| CrossRef
- Mehrabi M, Jangjoo A, Tavoosi H, Kahrom M, Kahrom H. Long-term outcome of Rives-Stoppa technique in complex ventral incisional hernia repair. World J Surg. 2010; 34: 1696–1701. View Full Text| PubMed| CrossRef
- Chrysos E, Athanasakis E, Saridaki Z, et al. Surgical repair of incisional ventral hernias: tension-free technique using prosthetic materials (expanded polytetrafluoroethylene Gore-Tex Dual Mesh). Am Surg. 2000; 66: 679–682. PubMed
- Diaz JJ Jr, Gray BW, Dobson JM, et al. Repair of giant abdominal hernias: does the type of prosthesis matter? Am Surg. 2004; 70: 396–401; discussion 401–402.
- Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990; 86: 519–526. View Full Text| PubMed| CrossRef
- Vargo D. Component separation in the management of the difficult abdominal wall. Am J Surg. 2004; 188: 633–637. PubMed| CrossRef
- de Vries Reilingh TS, van Goor H, Rosman C, et al. “Components separation technique” for the repair of large abdominal wall hernias. J Am Coll Surg. 2003; 196: 32–37. PubMed| CrossRef
- Szczerba SR, Dumanian GA. Definitive surgical treatment of infected or exposed ventral hernia mesh. Ann Surg. 2003; 237: 437–441. View Full Text| PubMed| CrossRef
- Lindsey JT. Abdominal wall partitioning (the accordion effect) for reconstruction of major defects: a retrospective review of 10 patients. Plast Reconstr Surg. 2003; 112: 477–485. View Full Text| PubMed| CrossRef
- Hadad I, Small W, Dumanian GA. Repair of massive ventral hernias with the separation of parts technique: reversal of the ‘lost domain’. Am Surg. 2009; 75: 301–306. PubMed
- Alaedeen DI, Lipman J, Medalie D, Rosen MJ. The single-staged approach to the surgical management of abdominal wall hernias in contaminated fields. Hernia. 2007; 11: 41–45. PubMed| CrossRef
- Candage R, Jones K, Luchette FA, Sinacore JM, Vandevender D, Reed RL 2nd. Use of human acellular dermal matrix for hernia repair: friend or foe? Surgery. 2008; 144: 703–709; discussion 709–711.
- Gupta A, Zahriya K, Mullens PL, Salmassi S, Keshishian A. Ventral herniorrhaphy: experience with two different biosynthetic mesh materials, Surgisis and Alloderm. Hernia. 2006; 10: 419–425. PubMed| CrossRef
- Limpert JN, Desai AR, Kumpf AL, Fallucco MA, Aridge DL. Repair of abdominal wall defects with bovine pericardium. Am J Surg. 2009; 198: e60–e65. PubMed| CrossRef
- Jin J, Rosen MJ, Blatnik J, et al. Use of acellular dermal matrix for complicated ventral hernia repair: does technique affect outcomes? J Am Coll Surg. 2007; 205: 654–660.
- Blatnik J, Jin J, Rosen M. Abdominal hernia repair with bridging acellular dermal matrix—an expensive hernia sac. Am J Surg. 2008; 196: 47–50. PubMed| CrossRef
- Patel KM, Nahabedian MY, Gatti M, Bhanot P. Indications and outcomes following complex abdominal reconstruction with component separation combined with porcine acellular dermal matrix reinforcement. Ann Plast Surg. 2012; 69: 394–398. View Full Text| PubMed| CrossRef
- Kim H, Bruen K, Vargo D. Acellular dermal matrix in the management of high-risk abdominal wall defects. Am J Surg. 2006; 192: 705–709. PubMed| CrossRef
- Kolker AR, Brown DJ, Redstone JS, Scarpinato VM, Wallack MK. Multilayer reconstruction of abdominal wall defects with acellular dermal allograft (AlloDerm) and component separation. Ann Plast Surg. 2005; 55: 36–41; discussion 41–42.
- Satterwhite TS, Miri S, Chung C, Spain D, Lorenz HP, Lee GK. Outcomes of complex abdominal herniorrhaphy: experience with 106 cases. Ann Plast Surg. 2012; 68: 382–388. View Full Text| PubMed| CrossRef
- Satterwhite TS, Miri S, Chung C, Spain DA, Lorenz HP, Lee GK. Abdominal wall reconstruction with dual layer cross-linked porcine dermal xenograft: the “Pork Sandwich” herniorraphy. J Plast Reconstr Aesthet Surg. 2012; 65: 333–341. PubMed| CrossRef
- Nasajpour H, LeBlanc KA, Steele MH. Complex hernia repair using component separation technique paired with intraperitoneal acellular porcine dermis and synthetic mesh overlay. Ann Plast Surg. 2011; 66: 280–284. View Full Text| PubMed| CrossRef
- Kanaan Z, Hicks N, Weller C, Bilchuk N, Galandiuk S, Vahrenhold C, Yuan X, Rai S. Abdominal wall component release is a sensible choice for patients requiring complicated closure of abdominal defects. Langenbecks Arch Surg. 2011; 396: 1263–1270. PubMed| CrossRef
- Lee EI, Chike-Obi CJ, Gonzalez P, et al. Abdominal wall repair using human acellular dermal matrix: a follow-up study. Am J Surg. 2009; 198: 650–657. PubMed| CrossRef
- Ueno T, Pickett LC, de la Fuente SG, Lawson DC, Pappas TN. Clinical application of porcine small intestinal submucosa in the management of infected or potentially contaminated abdominal defects. J Gastrointest Surg. 2004; 8: 109–112. PubMed| CrossRef
- Ghazi B, Deigni O, Yezhelyev M, Losken A. Current options in the management of complex abdominal wall defects. Ann Plast Surg. 2011; 66: 488–492. View Full Text| PubMed| CrossRef
- Byrnes MC, Irwin E, Carlson D, Campeau A, Gipson JC, Beal A, Croston JK. Repair of high-risk incisional hernias and traumatic abdominal wall defects with porcine mesh. Am Surg. 2011; 77: 144–150. PubMed
- Shah BC, Tiwari MM, Goede MR, Eichler MJ, Hollins RR, McBride CL, Thompson JS, Oleynikov D. Not all biologics are equal! Hernia. 2011; 15: 165–171. PubMed| CrossRef
- Ko JH, Salvay DM, Paul BC, Wang EC, Dumanian GA. Soft polypropylene mesh, but not cadaveric dermis, significantly improves outcomes in midline hernia repairs using the components separation technique. Plast Reconstr Surg. 2009; 124: 836–847. View Full Text| PubMed| CrossRef
- Joels CS, Vanderveer AS, Newcomb WL, et al. Abdominal wall reconstruction after temporary abdominal closure: a ten-year review. Surg Innov. 2006; 13: 223–230.
- Blatnik JA, Harth KC, Aeder MI, Rosen MJ. Thirty-day readmission after ventral hernia repair: predictable or preventable? Surg Endosc. 2011; 25: 1446–1451.
- Diaz JJ Jr, Guy J, Berkes MB, Guillamondegui O, Miller RS. Acellular dermal allograft for ventral hernia repair in the compromised surgical field. Am Surg. 2006; 72: 1181–1187; discussion 1187–1188.
- Tolino MJ, Tripoloni DE, Ratto R, Garcia MI. Infections associated with prosthetic repairs of abdominal wall hernias: pathology, management and results. Hernia. 2009; 13: 631–637. PubMed| CrossRef
- Ko JH, Wang EC, Salvay DM, Paul BC, Dumanian GA. Abdominal wall reconstruction: lessons learned from 200 “components separation” procedures. Arch Surg. 2009; 144: 1047–1055. View Full Text| PubMed| CrossRef
- Stremitzer S, Bachleitner-Hofmann T, Gradl B, Gruenbeck M, Bachleitner-Hofmann B, Mittlboeck M, Bergmann M. Mesh graft infection following abdominal hernia repair: risk factor evaluation and strategies of mesh graft preservation. A retrospective analysis of 476 operations. World J Surg. 2010; 34: 1702–1709. View Full Text| PubMed| CrossRef
- Diaz JJ Jr, Conquest AM, Ferzoco SJ, et al. Multi-institutional experience using human acellular dermal matrix for ventral hernia repair in a compromised surgical field. Arch Surg. 2009; 144: 209–215. PubMed| CrossRef
- Robertson JD, de la Torre JI, Gardner PM, Grant JH 3rd, Fix RJ, Vasconez LO. Abdominoplasty repair for abdominal wall hernias. Ann Plast Surg. 2003; 51: 10–16. View Full Text| PubMed| CrossRef
- Yegiyants S, Tam M, Lee DJ, Abbas MA. Outcome of components separation for contaminated complex abdominal wall defects. Hernia. 2012; 16: 41–45. PubMed| CrossRef
- Saulis AS, Dumanian GA. Periumbilical rectus abdominis perforator preservation significantly reduces superficial wound complications in “separation of parts” hernia repairs. Plast Reconstr Surg. 2002; 109: 2275–2280; discussion 2281–2272.
- Garvey PB, Bailey CM, Baumann DP, Liu J, Butler CE. Violation of the rectus complex is not a contraindication to component separation for abdominal wall reconstruction. J Am Coll Surg. 2012; 214: 131–139. PubMed| CrossRef
- DiCocco JM, Magnotti LJ, Emmett KP, et al. Long-term follow-up of abdominal wall reconstruction after planned ventral hernia: a 15-year experience. J Am Coll Surg. 2010; 210: 686–695, 695–688. PubMed| CrossRef
- Sailes FC, Walls J, Guelig D, Mirzabeigi M, Long WD, Crawford A, Moore JH Jr, Copit SE, Tuma GA, Fox J. Synthetic and biological mesh in component separation: a 10-year single institution review. Ann Plast Surg. 2010; 64: 696–698. View Full Text| PubMed
- Rios A, Rodriguez JM, Munitiz V, Alcaraz P, Pérez D, Parrilla P. Factors that affect recurrence after incisional herniorrhaphy with prosthetic material. Eur J Surg. 2001; 167: 855–859. PubMed| CrossRef
- Rosen MJ, Jin J, McGee MF, Williams C, Marks J, Ponsky JL. Laparoscopic component separation in the single-stage treatment of infected abdominal wall prosthetic removal. Hernia. 2007; 11: 435–440. PubMed| CrossRef
- Milburn ML, Shah PK, Friedman EB, et al. Laparoscopically assisted components separation technique for ventral incisional hernia repair. Hernia. 2007; 11: 157–161. PubMed| CrossRef
- Albright E, Diaz D, Davenport D, Roth JS. The component separation technique for hernia repair: a comparison of open and endoscopic techniques. Am Surg. 2011; 77: 839–843. PubMed
- Giurgius M, Bendure L, Davenport DL, Roth JS. The endoscopic component separation technique for hernia repair results in reduced morbidity compared to the open component separation technique. Hernia. 2012; 16: 47–51. PubMed| CrossRef
- Butler CE, Campbell KT. Minimally invasive component separation with inlay bioprosthetic mesh (MICSIB) for complex abdominal wall reconstruction. Plast Reconst Surg. 2011; 128: 698–709. View Full Text| PubMed| CrossRef
- Ghali S, Turza KC, Baumann DP, Butler CE. Minimally invasive component separation results in fewer wound-healing complications than open component separation for large ventral hernia repairs. J Am Coll Surg. 2012; 214: 981–989. PubMed| CrossRef
- Krpata DM, Blatnik JA, Novitsky YW, Rosen MJ. Posterior and open anterior components separations: a comparative analysis. Am J Surg. 2012; 203: 318–322. PubMed| CrossRef
- Novitsky YW, Elliott HL, Orenstein SB, Rosen MJ. Transversus abdominis muscle release: a novel approach to posterior component separation during complex abdominal wall reconstruction. Am J Surg. 2012; 204: 709–716. PubMed| CrossRef
- Rosenberger LH, Politano AD, Sawyer RG. The surgical care improvement project and prevention of post-operative infection, including surgical site infection. Surg Infect (Larchmt). 2011; 12: 163–168.
- Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996; 334: 1209–1215. View Full Text| PubMed| CrossRef
- Christy MR, Apostolides J, Rodriguez ED, Manson PN, Gens D, Scalea T. The component separation index: a standardized biometric identity in abdominal wall reconstruction. Eplasty. 2012; 12: 169–176.
- Dragu A, Klein P, Unglaub F, Polykandriotis E, Kneser U, Hohenberger W. Tensiometry as a decision tool for abdominal wall reconstruction with component separation. World J Surg. 2009; 33: 1174–1180. PubMed| CrossRef
- Mazzocchi M, Dessy LA, Sorvillo V, Di Ronza S, Scuderi N. A study of intraabdominal pressure modification in “component separation” technique for repair of incisional hernia. Ann Ital Chir. 2010; 81: 433–437. PubMed
- Buck DW 2nd, Steinberg JP, Fryer J, Dumanian GA. Operative management of massive hernias with associated distended bowel. Am J Surg. 2010; 200: 258–264. PubMed| CrossRef
- Mazzocchi M, Dessy LA, Ranno R, Carlesimo B, Rubino C. “Component separation” technique and panniculectomy for repair of incisional hernia. Am J Surg. 2011; 201: 776–783. PubMed| CrossRef