Pancreatic Necrosis, Surgical Management of
Published 2017
Citation: J Trauma. 83(2):316-327, August 2017
Authors
Mowery, Nathan T. MD; Bruns, Brandon R. MD; MacNew, Heather G. MD; Agarwal, Suresh MD; Enniss, Toby M. MD; Khan, Mansoor MBBS, PhD; Guo, Weidun Alan MD, PhD; Cannon, Jeremy W. MD; Lissauer, Matthew E. MD; Duane, Therese M. MD; Hildreth, Amy N. MD; Pappas, Peter A. MD; Gries, Lynn M. MD; Kaiser, Meghann MD; Robinson, Bryce R.H. MD
Author Information
From the Department of Surgery, Wake Forest University (N.T.M., A.N.H.), Winston-Salem, North Carolina; Department of Surgery, University of Maryland (B.R.B.), Baltimore, Maryland; Memorial University Medical Center, Mercer University School of Medicine (H.G.M.), Savannah, Georgial; University of Wisconsin (S.A.), Madison, Wisconsin; University of Utah (T.E.), Salt Lake City, Utah; Imperial College Healthcare NHS Trust (M.K.), London, England; SUNY-University at Buffalo (W.A.G.), Buffalo, New York; University of Pennsylvania (J.W.C.), Philadelphia, Pennsylvania; Rutgers-Robert Wood Johnson Medical School (M.L.), New Brunswick, New Jersey; JPS Health Network (T.M.D.), Fort Worth, Texas; University of Central Florida (P.P.), Holmes Regional Medical Center, Melbourne, Florida; University of Arizona (L.G.), Tucson, Arizona; Greenville Health System (M.K.), Greenville, South Carolina; and University of Washington (B.R.), Seattle, Washington.
Submitted: November 7, 2016, Revised: December 28, 2016, Accepted: January 18, 2017, Published online: April 27, 2017.
This work was presented at The Eastern Association for the Surgery of Trauma 27th Annual Scientific Assembly, January 14–18, 2014, in Naples, Florida.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
Address for reprints: Nathan T. Mowery, MD, Department of Surgery, Wake Forest University, Medical Center Medical Center Boulevard, Winston-Salem, NC 27157; email: nmowery@wakehealth.edu.
Overview
Pancreatic parenchymal and/or peripancreatic tissue necrosis (i.e., necrotizing pancreatitis) occurs in approximately 15% of patients with acute pancreatitis (AP) and confers substantial additional morbidity and mortality. Infection occurs in one third of patients with necrosis.[1-4] Mortality is approximately 15% in patients with necrotizing pancreatitis and up to 30% in those with infected necrosis.
Historically, intervention was required for patients with infected pancreatic necrosis and, less commonly, in patients with symptomatic sterile necrosis. Manifestations of symptomatic pancreatic necrosis can range from persistent fevers, intractable nausea, lethargy, and failure to thrive to more problematic consequences, particularly biliary or gastric outlet obstruction. Although open surgical debridement has been the traditional treatment, it has been suspected that the physiologic stress of open surgical debridement increases complications. Efforts to minimize the stress of intervention have contributed significantly to the evolution of management of pancreatitis over the past 30 years.
Over the last decade, there have been substantial developments in the treatment of necrotizing pancreatitis. Most of this progress has been a result of the proliferation of minimally invasive treatment approaches for drainage and evacuation of pancreatic necrosis, including image guided radiological, endoscopic trans-gastric, laparoscopic, and video-assisted retroperitoneal endoscopic (VARD) techniques. Literature over the last 20 years has identified patient populations that have benefited from these new interventions and have further improved patient outcomes.
As the field of acute care surgery evolves, its practitioners are increasingly being called upon to be the primary service managing these complex patients. The timing and choice of intervention is a decision that often must be made outside of hours of optimal resource availability and are decisions that can result in increased complications. The purpose of this practice management guideline (PMG), which is sponsored by the Eastern Association for the Surgery of Trauma, is to provide evidence-based recommendations to be used to direct the decision-making processes related to the surgical management of patients with pancreatic necrosis. This guideline has been developed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework that was adopted by Eastern Association for the Surgery of Trauma in 2012.[5]

TABLE 1: Study PICO Questions
Objectives
This PMG addresses three population, intervention, comparators, and outcome (PICO) questions that would be answered by the available evidence to guide surgical treatment decisions in caring for patients with pancreatitis-associated necrosis[6] (Table 1).
Inclusion Criteria for this Review
Study Types
In constructing the GRADE recommendations, only studies with comparison groups were included. This included randomized controlled trials, prospective observational or retrospective studies, and case control studies. Additional support was based on case studies to give a complete picture of the current literature. Meta-analyses, letters, and reviews containing no original data or comments were excluded.
Participant Types
We included studies of adult patients without restricting sex, ethnicity, or degree of comorbidity. Only studies pertaining to the treatment of hospitalized patients with necrotizing pancreatitis were included.
In the more recent literature, the distinction between infected and sterile necrosis has been obscured. Since the included studies focused on surgical intervention for pancreatic necrosis, and the traditional treatment of infected necrosis was surgery, many of the included patients carried the diagnosis of infected necrosis. How the diagnosis of infected necrosis was reached varied greatly, and all included studies have patients that were labeled as infected. More recent studies do not make the distinction between infected and sterile necrosis as they move through the algorithm but rather discuss delaying intervention for both until maturation of the process. Given this evolution in treatment, we did not exclude or limit the included patients or studies based on the presence of infection.
Intervention Type
The first PICO question examined the operative timing. The definition of early and late surgical intervention has evolved over time and varies from study to study. Although prospective randomized data do exist on the subject, trying to find multiple studies required the inclusion of subsets of larger studies. Three separate analyses were performed to examine the potential clinically relevant timeframes. Analysis of greater and less than 72 hours was used to look at a period that defined very early intervention that was common in the early 1980s and is still used by some surgeons in infected necrosis. An intermediate value of 12 days for the cutoff of early/late was included to address the numerous studies that have used the 10-day to 14-day mark as a timeframe for surgical intervention. Finally, a 30-day definition of early and late was used due to the recent literature.
The second PICO question compares surgical intervention to the two main alternatives, percutaneous and endoscopic drainage (DEN). There is limited data directly comparing surgery to each of the alternative methods of drainage but some indirect comparisons can be made. In the case of percutaneous methods, many of the minimally invasive surgical techniques include percutaneous drainage (PCD) as a preoperative intervention. A certain percentage of the patients have resolution of their disease with percutaneous intervention alone giving an opportunity for additional comparison groups. The other complicating factor is that surgery is used as a salvage procedure for both failed percutaneous intervention and DEN. This selects the surgical population for poor outcomes.
Finally, the third PICO question addressing open versus minimally invasive intervention has small numbers but with the most uniform outcomes.
Outcome Measure Types
Outcomes were chosen by the committee and the uniformity of their presence in the literature was examined. Outcomes were rated in importance from 1 to 9, with scores of 7 to 9 representing critical outcomes. The following outcomes were considered by the committee: length of stay, intensive care unit length of stay, cost and ventilator-free days. However, all of these criteria were deemed noncritical for the decision-making process within the GRADE framework. Also, the available literature did not provide sufficient or consistent measurements across the studies, specifically if the onset of related conditions, such as renal or respiratory failure, occurred before or after surgical intervention. Mortality was deemed a critical outcome for the decision-making process across all PICO questions, and this was chosen as the primary outcome measure.
Review Methods
Search Strategy
A computerized search of the National Library of Medicine MEDLINE database was undertaken using the Entrez interface. English language citations during the period of January 1980 through December 2014 using the primary search strategy:
Pancreatic necrosectomy[mh] AND humans[mh] AND English NOT (reviews[pt] OR letter[pt] OR comment[pt] OR news[pt]).
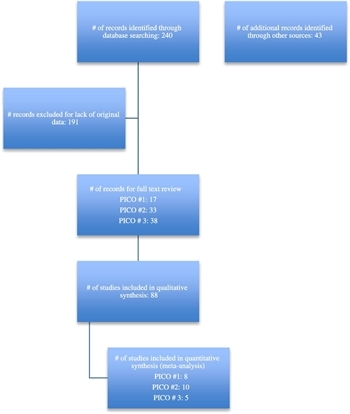
Figure 1: PRISMA diagram.
The -related articles algorithm was also used to identify additional articles similar to the items retrieved by the primary strategy. Of the 283 articles identified by these two techniques, those dealing with either prospective or retrospective studies examining the management of pancreatic necrosis were selected, comprising 88 institutional studies evaluating diagnosis and management of adult patients with pancreatic necrosis (Fig. 1). For a complete listing of the articles reviewed and considered for inclusion please see the table in the supplemental digital content (http://links.lww.com/TA/B26). The articles were reviewed by a group of nine surgeons who collaborated to produce this PMG. When discrepancy existed about inclusion or data extracted, the majority among the three reviewers to read each article was used. Meta-analysis was performed using REVMAN 5 online data analysis (The Cochrane Collaboration, Informatics & Knowledge Management Department, London, United Kingdom) to give an overall point estimate and confidence interval for the effect size that the intervention had on the outcome of interest. Evidence tables were created by collating the committee members reviews and GradePRO online software (www.Gradepro.org). The recommendations flowed from the outcomes of the meta-analysis and wording was reached by critical evaluation of several drafts of the recommendations. In points of disagreement the majority vote ruled.
Results Obtained for Operative Timing in Pancreatic Necrosis (PICO 1)
PICO Question 1
In adult patients with pancreatic necrosis (P) does early surgery (I) compared with late surgery (C) decrease mortality rates (O)?
Qualitative Synthesis
Data was divided into three different periods to examine the timing of surgical intervention. This also allowed an easier fit for the data rather than forcing studies to supply information that was never part of their original study design. All three periods selected (72 hours, 12 days and 30 days) favored delaying surgery.

Figure 2: (A) Timing of operative intervention, early (<72 hours) or late (>72 hours), meta-analysis. (B) Timing of operative intervention, early (≤12 days) or late (>12 days), meta-analysis. (C) Timing of operative intervention, early (≤30 days) or late (>30 days), meta-analysis.
The data for early surgery, defined at less than 72 hours, are limited (Fig. 2A). This is the one period that has prospective randomized data available.[7] In this study, early necrosectomy (within the first 72 hours after onset of AP) produced a higher morbidity and mortality than delayed intervention after at least 12 days. The mortality in the early surgery group was more than double the late surgery group (56% vs. 27%), and although this did not reach statistical significance (due to the study being stopped early for safety concerns), the odds ratio for mortality was 3.4 times higher in the early surgical group. These authors did not divide the study population into infected and sterile collections, nor did they use infected necrosis as an indication for surgery. When examining just the infected collections, the mortality for early (< 72 hours) surgical intervention was 73% compared with 29% for those whose surgery was delayed to hospital day 12 (p < 0.05).[7] Hartwig showed a retrospective similar fourfold increase if early surgery was attempted.[8]
All six articles included in the 14-day to 21-day cutoff independently favor later surgical intervention (Fig. 2B). This model predicts nearly a fivefold increase in the mortality with early intervention. Although there exists some slight variability in the study design concerning the actual day of surgical intervention, all the studies fall in the approximate range of 2 weeks.
There were four articles that included data about delaying necrosectomy to 30 days (Fig. 2C). The survival benefit for delayed surgical intervention was nearly fourfold.
Quantitative Synthesis
Before pooling study data, we assessed methodological and clinical heterogeneity across the studies and found variability in how the intervention and comparator were defined. No two studies used the same timeframe. We attempted to correct for that by running three separate meta-analyses. We found that early surgical intervention was associated with increased mortality rates with a risk ratio (RR) as much as 4.88 (95% confidence interval [CI], 3.53–6.73) in the 12-day group.
Grading the Evidence

TABLE 2: (A) Timing of Operative Intervention, Early (<72 h) or late (>72 h), Evidence Profile
When examining the question of immediate necrosectomy compared to delaying 72 hours, the overall data were considered low quality. There were no serious risks of bias, inconsistency, indirectness, or publication bias was found. There was one randomized study, and the results agreed with the observational data. However, imprecision was observed since the studies were small, and there were only two studies using that timeframe. Despite imprecision and relatively low quality of evidence the magnitude of effect was large with an RR of 3.71 (Table 2A).

TABLE 2: (B) Timing of Operative Intervention, Early (≤12 d) or Late (>12 d), Evidence Profile
When using a definition of 12 days to differentiate operative timing, there were more data but also higher imprecision. When assessing the outcome no serious risk of bias, indirectness, or publication, bias was found. However, very serious inconsistency was noted since the studies had variability on the exact timing of intervention. Starting from observational studies (which are considered low quality), we then rated down for inconsistency. Therefore, the overall quality of evidence was very low (Table 2B).

TABLE 2: (C) Timing of Operative Intervention, Early (≤30 d) or Late (>30 d), Evidence Profile
Finally, with a 30-day definition of early and late intervention and assessing the outcome of reduced mortality rates, no serious risk of bias, indirectness was found. There were only observational studies on the topic, which downgraded the overall grade. However, severe imprecision was noted since there was variation in the intervention performed at 30 days. Therefore, the overall quality of evidence was graded as low (Table 2C).
Discussion for Operative Timing in Pancreatic Necrosis (PICO 1)
Traditionally, indications for intervention within weeks of the onset of AP included clearly infected acute necrotic collections associated with clinical deterioration and signs of sepsis. In contrast, clinical deterioration despite maximum medical support, in patients without documented infection, has not been an indication for surgical drainage or necrosectomy.[9][10] Studies have looked at using surgical intervention in these patients as a salvage operation and found that it did not have the desired lifesaving effect.[3][9] One exception may be abdominal compartment syndrome, where surgical decompression may be lifesaving.
In addition to the presence of multiorgan failure and a high APACHE II score, early surgery has been shown to be an independent predictor of poor outcome in acute necrotizing pancreatitis.[4] Several case series also underscore that mortality decreases when interventions are postponed.[9][13] As advocated in guidelines by the International Association of Pancreatology in 2002,[3] delay in open surgery for at least 3 weeks to 4 weeks leads to lower morbidity and mortality rates. With delay in intervention, demarcation of necrotic from vital tissue occurs, so that if necrosectomy is performed, resection of vital tissue is minimized. This leads to better long-term endocrine and exocrine function and a reduction in postoperative adverse events.[3] The advantage of delayed surgery is especially evident in series in which timing of intervention was changed within a single institution.[10][11][13][14] When emergency interventions are needed for perforated viscus, acute bleeding, fistula to or obstruction of a viscus, and abdominal compartment syndrome, drainage or debridement of necrosis is not required at the same setting.[3]
Recommendation
The panel determined that the quality of evidence was low overall; we also considered that most patients would place a high value on the potential 50% reduction in mortality seen with delaying surgery. Although the exact number of how long to delay is in question, it would appear that delaying at least 12 days and potentially 30 days would lead to additional decreases in mortality. This allows for a strong recommendation due to patient preference. Thus, in adult patients with pancreatic necrosis, we recommend that pancreatic necrosectomy should be delayed until at least day 12, as opposed to earlier necrosectomy.
Results Obtained for Adjuvant Therapy Use in Pancreatic Necrosis (PICO 2)
PICO Question 2
In adult patients with pancreatic necrosis (P), does primary surgical intervention (I) compared with PCD (C) or DEN (C) decrease mortality rates (O)?
Qualitative Synthesis
The use of less invasive techniques allows surgical debridement to be deferred or avoided altogether.[4][7-9][12] The two major adjuvant treatments for necrosectomy are PCD and DEN or some combination of them both.
Percutaneous Drain Versus Surgery
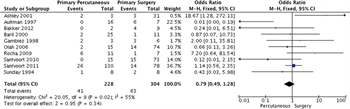
Figure 3: Primary percutaneous vs. primary surgical intervention with mortality as the outcome, meta-analysis.
Percutaneous catheter drainage originally played an adjuvant role in draining residual collections after open necrosectomy[15] but is increasingly used in a step-up approach to defer or obviate the need for surgery or as an adjunct to endoscopic necrosectomy.[16] It has been shown that placement of drains can delay surgery and allow for decreased mortality.[13] A systematic review of PCD as primary treatment for pancreatic necrosis included 11 studies involving 384 patients.[17] Seventy percent of patients had infected necrosis and an average of two separate catheters were placed, with an overall success rate of 56%. In two prospective studies, the clinical success of PCD alone was found to be 33% and 35%.[16][18] Adverse events, such as external fistulae, occur in up to 27% of patients. We found 10 studies that had comparison groups of primary PCD compared with primary surgical intervention. Although many of the studies were small and not powered to show a mortality difference, they did show safety with a significant percentage of patients having complete resolution of their process with PCD only (Fig. 3).
Endoscopic Versus Surgery
A full analysis using GRADE methodology comparing primary endoscopic intervention to surgical intervention is not possible. Although numerous case series suggesting the feasibility and safety of endoscopic intervention exist, there is only one study comparing the two interventions. There is one prospective randomized trial with a small sample size making definitive conclusions challenging. The Dutch Pancreatitis Study Group compared DEN with minimally invasive surgical necrosectomy or, if not feasible, open necrosectomy.[19] Twenty patients completed randomization with 10 in each group. The study showed superiority of DEN over surgical necrosectomy with regard to inflammatory markers and a composite end point of major complications. New-onset organ failure occurred significantly less frequently with endoscopic necrosectomy (0% vs. 50%, p = 0.03), as did pancreatic fistulas (10% vs. 70%, p = 0.02). There was an insignificant trend toward lower mortality with DEN compared with surgery (10% vs. 40%). The authors concluded that DEN was superior over surgical necrosectomy for infected necrosis. The drawback was that endoscopic patients required a median of three procedures to accomplish what a surgical necrosectomy did in one (p = 0.007).
In reviewing retrospective studies, overall morbidity was 27% and mortality was 5%, which is lower than that in most surgical series.[20][21] Success rates are seen as high as 80% to 90% with multiple sessions (median, 3 to 6). However, it should be noted that many of the patients included in these studies did not require preintervention admission to an intensive care unit, and the incidence of infected necrosis was lower than most surgical studies. These observations reinforce the concept that it is necessary to consider patient characteristics when comparing different reports and techniques.
Combined Percutaneous and Endoscopic
Advantages of PCD include widespread availability, access by transperitoneal and retroperitoneal approaches to the left and right sides of the abdomen and pelvis, ability to place multiple catheters, and the ability to flush catheters between procedures. However, a major limitation is the development, in at least 20% of patients, of pancreaticocutaneous fistulae, some of which do not close because of communication of the drain with an upstream disconnected pancreatic duct.[22][25] Combining a percutaneous approach with endoscopic transmural drainage can prevent external fistulae and avoid repetitive endoscopic interventions to perform direct necrosectomy.[26] Irrigation through the percutaneous approach with egress through the transmural fistula results in a form of debridement. In case-control series from a single center, the combined percutaneous-endoscopic approach has been shown to increase the rate of nonsurgical resolution and result in a decrease in hospitalization, time to drain removal, number of CT scans, and number of drains compared to PCD alone.[26][27]
Quantitative Synthesis
In examining the data, we were unable to show a difference in the different types of drainage procedures in terms of their effect on mortality (Fig. 3). Other end points, such as number of procedures, were not published in enough studies to generate a comment. Of note, the I2 statistic was 55%, falling into the “moderate” heterogeneity category, indicating that the studies may not be comparable.
Grading the Evidence

TABLE 3: Primary Percutaneous Versus Primary Surgical Intervention With Mortality as the Outcome, Evidence Profile
With the use of the GRADE framework for assessing the outcome of reduced mortality rates, no serious risk of bias, inconsistency or indirectness. However, severe imprecision was noted since the studies were small and the confidence intervals were large. Starting from observational studies we then rated down for imprecision. Therefore, the overall quality of evidence was very low (Table 3).
Discussion for Adjuvant Therapy Use in Pancreatic Necrosis (PICO 2)
In addition to the surgery for the treatment of pancreatic necrosis, a variety of nonsurgical methods have been described. The issue is that these studies involve heterogeneous patient populations, definitions of infected necrosis, and techniques. As a result, outcomes are not directly comparable outside of randomized trials. The effect of nonsurgical methods when compared with surgery is variable, and definitive mortality decreases have not been seen. Advantages of nonsurgical approaches include a reduction in systemic complications after intervention and a lower risk of developing new organ failure.[16][28] Local adverse events including bleeding and fistula seem to be slightly increased in some retrospective studies when minimally invasive treatment regimens are used, although this finding may reflect a difference in the definition of adverse events or represent a learning curve associated with early results.[16][29-31]
PCD can be used as primary therapy, as an adjunct to other techniques, and as salvage management of residual necrotic or infected collections.[16][32-33] Approaches include transperitoneal or retroperitoneal placement of 12-Fr to 30-Fr catheters. Retroperitoneal approach is generally preferred because it avoids contamination and enteric leaks and facilitates a step-up approach.[17][34] In general, at least one separate catheter is required for each collection, which can result in multiple catheters per patient.[35] The optimal size, number of drains, and management of drains when PCD is used are unknown.
DEN is performed by passing a flexible endoscope transorally then transmurally into the necrotic cavity; mechanical debridement and lavage are performed. DEN requires a collection to be located within several centimeters of the gastric or duodenal lumen. Typically, three to six sessions are necessary to completely debride the cavity.
In one multicenter randomized study comparing endoscopic intervention to surgical debridement, there were nonsignificant mortality differences. This single trial is supported by case series that show safety of DEN, some reporting mortality numbers that would be lower than historical surgical series. The issue would be that newer minimally invasive surgical debridements have markedly lower morbidity and mortality than the open early surgical interventions of the past. A definitive statement about the benefits of one approach over the other cannot be reached.
One of the limitations of the transgastric endoscopic approach is the location of the target collection. Central collections are almost always accessible, but not left-sided and flank collections. As a result, the VARD procedure and the other percutaneous retroperitoneal approaches are likely to continue to have a significant role to play when there is no close abutment of the collection to the stomach or duodenum.
Recommendation
The overall quality of evidence for the topic is very low. PCD and endoscopic debridement may have a role in the management of pancreatic necrosis at times as a definitive treatment. It certainly has value as a means to delay surgical intervention until a time that it is safe. Pursing PCD or DEN as primary therapy has a questionable effect on mortality but has been shown to increase the number of total procedures. This would have a corresponding effect on hospital days and potentially health care costs. Numerous studies, including our analysis, show equivalence in the different types of interventions suggesting health care teams have the ability to tailor care to the individual patient. This allows for a conditional recommendation based on the ability to choose which options fit the patient best.
RECOMMENDATION: In adult patients within the first 30 days of symptoms with infected necrotic collections, we conditionally recommend surgical debridement only if the patient fails to improve after radiologic or DEN. After 30 days, all 3 means of drainage have equivalent results.
Results Obtained for Surgical Approach to Pancreatic Necrosis (PICO 3)
PICO Question 3
In adult patients undergoing surgery for pancreatic necrosis (P) do minimally invasive approaches (I) compared with open approaches (C) decrease the mortality rate (O)?
Qualitative Synthesis
Open necrosectomy is associated with relatively high morbidity (34%–95%) and mortality ranging from 6% to 25%.[11][14-16][31][36-41] Historically there are differences in surgical techniques when looking at open necrosectomy. In the 6 studies included in our analysis, all patients in the open group received a variation on debridement followed by closed post-operative lavage.
Minimally invasive approaches are thought to induce less stress than open surgery in already critically ill patients.[29][33][42-43] The step-up approach is currently supported with phase 1 feasibility and phase 2 prospective safety and efficacy data.[18][43-44] There is one randomized control trial (PANTER trial) evaluating if the step-up approach is superior to open necrosectomy as first-line treatment for necrosis.[16] In this Dutch multi-institutional trial, 45 patients were randomized to immediate open necrosectomy, and 43 patients were randomized to the step-up approach. Baseline patient characteristics were similar in both groups. Outcomes were significantly better in the step-up group compared to the open surgery group. Death or major morbidity occurred in 40% of the step-up group compared with 69% of the open necrosectomy group; mortality was similar in both groups (19% vs. 16%). Twelve percent of patients in the step-up group experienced new onset multi-organ failure compared to 42% in the open necrosectomy group. Long-term morbidity including incisional hernia (7% vs. 24%), new-onset diabetes mellitus (16% vs. 38%), pancreatic enzyme use (7% vs. 33%), and cost of care were all significantly lower in the step-up group. Notably, 35% of patients in the step-up group did not require necrosectomy.
Quantitative Synthesis
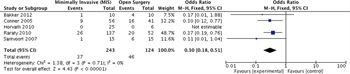
Figure 4: Surgical approach with mortality as the outcome, meta-analysis.
Before pooling study data, we assessed methodological and clinical heterogeneity across the studies and found variability in how the intervention and comparator were defined. We found that minimally invasive approach was associated with reduced mortality rates with an RR of 0.51 (95% CI, 0.32–0.81; [Fig. 4]). Of note, the I2 statistic was 0% indicating that the studies are comparable.
Grading the Evidence

TABLE 4: Surgical Approach With Mortality as the Outcome, Evidence Profile
No serious risk of bias, inconsistency, indirectness, or publication bias was found. However, severe imprecision was noted since the studies were small and the confidence intervals were large. There was one randomized trial on the topic and several large retrospective studies pertinent to the topic. Still, the overall quality of evidence was low (Table 4).
Discussion for Surgical Approach to Pancreatic Necrosis (PICO 3)
Open surgical debridement historically has been the standard treatment for infected necrosis and for symptomatic sterile walled of necrosis with the aim of complete removal of necrotic tissue. The oldest and most established approach includes open laparotomy or retroperitoneal flank incision with manual debridement. After necrosectomy, the abdomen may be left open or drains are placed for postoperative continuous lavage. The postoperative lavage technique was associated with less operations and a decrease in postoperative morbidity.[15] The technique of closed packing is mainly advocated by a single group.[36] In some cases of incomplete necrosectomy, percutaneous drains can be placed if needed to provide irrigation and to further debride postoperative collections (step-down approach). We chose to aggregate open necrosectomy into one cohort despite some differences in described surgical techniques. We felt comfortable doing this due to the fact that when comparing the principal approaches for open necrosectomy, there is consensus but limited data to support the claim that one intervention was better than the other.
An alternative to open initial necrosectomy is the step-up approach, using minimally invasive techniques to control necrosis, with definitive necrosectomy deferred or sometimes avoided altogether. The first step is usually PCD, preferably into the retroperitoneum via the left flank. Alternative routes are percutaneous transabdominal or endoscopic transluminal. The objective is to postpone or obviate the need for surgery. If drainage fails to control sepsis, the next step is taken, and debridement accomplished via VARD, sinus tract endoscopy, or DEN. These approaches are thought to induce less stress than open surgery in already critically ill patients.[29][33][42-43]
One potential issue would be the overlap in the surgical approach with the surgical timing. The step-up procedure is designed to push surgery out to 30 days when possible. Given our earlier PICO question that would favor a delayed surgical intervention, it is possible that the benefit seen in the step-up approach is due to the surgical timing rather than the operative approach. While there are case series showing early laparoscopic surgical interventions can be done safely[29][30][45-49] there are none that compare laparoscopic-assisted pancreatic debridement to open intervention.
The potential learning curve associated with this procedure must also be recognized. While the Dutch papers show impressive improvements in outcomes, they are small and have not been widely reproduced in other populations. While acute care surgeons have significant experience in managing ill patients with distorted anatomy, they may not have extensive experience with this disease process and approaching it from a retroperitoneal point of view. With the introduction of all new surgical techniques, one must acknowledge that outcomes may improve over time as the surgeon develops their skill set. No published literature on the subject addresses the outcomes as the beginning of the series when compared to the end of the series.
Recommendation
The overall quality of evidence was rated as low. The panel considered that most patients would place a high value on the potential three-fold reduction in postoperative organ failure and 50% reduction in mortality. This allows for a strong recommendation.
In adult patients with pancreatic necrosis, even documented infected necrosis, we recommend that patients undergo a step-up approach to surgical intervention. This includes aggressive use of percutaneous drains as a means to delay or even definitively treat necrosis which may be the real benefit of this surgical pathway rather than the actual surgical incision. This recommendation is based on low-quality evidence and is associated with significant patient benefit.
Future Investigations
Although it would appear that waiting for maturity of the pancreatic necrosis will improve mortality, the exact time is still not known. Further studies are awaited to evaluate the efficacy of various combinations of techniques, including endoscopic, PCD, and retroperitoneoscopic techniques. Additional studies comparing these modalities are in progress. The TENSION trial is a randomized controlled, parallel-group superiority multicenter trial that began in 2013.[15] Patients with (suspected) infected necrotizing pancreatitis with an indication for intervention and in whom both treatment modalities are deemed possible, will be randomized to either an endoscopic or a surgical step-up approach. The primary endpoint is a composite of death and major complications within 6 months after randomization.
Conclusion
Many different interventions in varying states of evolution are available for the treatment of pancreatic and peripancreatic necrosis. These interventions should be offered in the context of optimal intensive care and medical management and that a multidisciplinary approach is required in a center with specialized expertise.
Intervention is required primarily with clinical deterioration and for symptomatic patients, especially those with obstruction of a viscus. Specific scenarios, such as abdominal compartment syndrome or perforated viscus, require urgent surgery, but not necrosectomy. Interventions within the first few weeks for pancreatic necrosis are generally associated with poor outcomes and should be reserved for infected necrosis in a severely deteriorating patient and may not have the beneficial desired effect.

TABLE 5: Summary of Recommendations
Traditionally, the most widely used approach to infected necrosis has been open surgical necrosectomy. Most cases can now be managed using minimally invasive techniques in specialized centers with the appropriate expertise. Percutaneous catheter drainage, endoscopic, and laparoscopic methods are all feasible approaches for treating infected necrosis. Combination approaches may be useful in selected patients with extensive peripancreatic necrosis. Current evidence favors endoscopic necrosectomy or percutaneous catheter drainage early in an effort to delay surgery followed by a step-up approach necrosectomy if needed (Table 5).
The overriding principle of interventions for necrosis is that no single approach is optimal for all patients. The best approach is multimodal and adaptable to the individual patient to achieve the best outcomes.
Authorship
N.T.M. participated in the literature search, study design, data collection, data analysis, data interpretation, writing, and critical revision. B.R.B. participated in the data analysis, data interpretation, and critical revision. H.MN. participated in the data analysis, data interpretation, and critical revision. S.A. participated in the data analysis, data interpretation, and critical revision. T.E. participated in the data analysis, data interpretation, and critical revision. M.K. participated in the data analysis, data interpretation, and critical revision. W.A.G. participated in the data analysis, data interpretation, and critical revision. J.W.C. participated in the data analysis, data interpretation, and critical revision. M.L. participated in the data analysis, data interpretation, and critical revision. T.M.D. participated in the data analysis, data interpretation, and critical revision. A.N.H. participated in the data analysis, data interpretation, writing, and critical revision. P.P. participated in the data analysis, data interpretation, writing, and critical revision. L.G. participated in the data analysis, data interpretation, writing, critical revision. M.K. participated in the data analysis, data interpretation, and critical revision. B.R. participated in the writing and critical revision.
Disclosure
The authors declare no conflicts of interest.
References
- Banks PA, Freeman ML. Practice Parameters Committee of the American College of G. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101(10):2379–2400.
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139(3):813–820.
- Uhl W, Warshaw A, Imrie C, et al. IAP guidelines for the surgical management of acute pancreatitis. Pancreatology. 2002;2(6):565–573.
- van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, Boermeester MA, van Goor H, Delong CH, van Eijck CH, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141(4):1254–1263.
- Andrews JC, Schunemann HJ, Oxman AD, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation's direction and strength. J Clin Epidemiol. 2013;66(7):726–735.
- Kerwin AJ, Haut ER, Burns JB, et al. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S283–S287.
- Mier J, Leon EL, Castillo A, Robledo F, Blanco R. Early versus late necrosectomy in severe necrotizing pancreatitis. Am J Surg. 1997;173(2):71–75.
- Hartwig W, Maksan SM, Foitzik T, Schmidt J, Herfarth C, Klar E. Reduction in mortality with delayed surgical therapy of severe pancreatitis. J Gastrointest Surg. 2002;6(3):481–487.
- Besselink MG, Verwer TJ, Schoenmaeckers EJ, Buskens E, Ridwan BU, Visser MR, Nieuwenhuijs VB, Gooszen HG. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg. 2007;142(12):1194–1201.
- Papachristou GI, Takahashi N, Chahal P, Sarr MG, Baron TH. Peroral endoscopic drainage/debridement of walled-off pancreatic necrosis. Ann Surg. 2007;245(6):943–951.
- Wittau M, Scheele J, Golz I, Henne-Bruns D, Isenmann R. Changing role of surgery in necrotizing pancreatitis: a single-center experience. Hepatogastroenterology. 2010;57(102–103):1300–1304.
- Bhansali SK, Shah SC, Desai SB, Sunawala JD. Infected necrosis complicating acute pancreatitis: experience with 131 cases. Indian J Gastroenterol. 2003;22(1):7–10.
- Olah A, Belagyi T, Bartek P, Poharnok L, Romics L Jr. Alternative treatment modalities of infected pancreatic necrosis. Hepatogastroenterology. 2006;53(70):603–607.
- Reddy M, Jindal R, Gupta R, Yadav TD, Wig JD. Outcome after pancreatic necrosectomy: trends over 12 years at an Indian centre. ANZ J Surg. 2006;76(8):704–709.
- Werner J, Feuerbach S, Uhl W, Buchler MW. Management of acute pancreatitis: from surgery to interventional intensive care. Gut. 2005;54(3):426–436.
- van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boemeester MA, Delong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362(16):1491–1502.
- van Baal MC, van Santvoort HC, Bollen TL, Bakker OJ, Besselink MG, Gooszen HG, Dutch Pancreatitis Study Group. Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br J Surg. 2011;98(1):18–27.
- Horvath K, Freeny P, Escallon J, et al. Safety and efficacy of video-assisted retroperitoneal debridement for infected pancreatic collections: a multicenter, prospective, single-arm phase 2 study. Arch Surg. 2010;145(9):817–825.
- Bakker OJ, van Santvoort HC, van Brunschot S, Geskus RB, Besselink MG, Bollen TL, van Eijck CH, Fockens P, Hazebroek EJ, Nijmeijer RM, et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307(10):1053–1061.
- Seifert H, Biermer M, Schmitt W, et al. Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long-term follow-up (the GEPARD Study). Gut. 2009;58(9):1260–1266.
- Gardner TB, Coelho-Prabhu N, Gordon SR, et al. Direct endoscopic necrosectomy for the treatment of walled-off pancreatic necrosis: results from a multicenter U.S. series. Gastrointest Endosc. 2011;73(4):718–726.
- Lee MJ, Rattner DW, Legemate DA, et al. Acute complicated pancreatitis: redefining the role of interventional radiology. Radiology. 1992;183(1):171–174.
- Rotman N, Mathieu D, Anglade MC, Fagniez PL. Failure of percutaneous drainage of pancreatic abscesses complicating severe acute pancreatitis. Surg Gynecol Obstet. 1992;174(2):141–144.
- Aultman DF, Bilton BD, Zibari GB, McMillan RW, McDonald JC. Nonoperative therapy for acute necrotizing pancreatitis. Am Surg. 1997;63(12):1114–1117; discussion 1117–1118.
- Sunday ML, Schuricht AL, Barbot DJ, Rosato FE. Management of infected pancreatic fluid collections. Am Surg. 1994;60(1):63–67.
- Ross A, Gluck M, Irani S, Hauptmann E, Fotoohi M, Siegal J, Robinson D, Crane R, Kozarek R. Combined endoscopic and percutaneous drainage of organized pancreatic necrosis. Gastrointest Endosc. 2010;71(1):79–84.
- Gluck M, Ross A, Irani S, et al. Dual modality drainage for symptomatic walled-off pancreatic necrosis reduces length of hospitalization, radiological procedures, and number of endoscopies compared to standard percutaneous drainage. J Gastrointest Surg. 2012;16(2):248–256; discussion 256–247.
- Babu BI, Sheen AJ, Lee SH, O'Shea S, Eddleston JM, Siriwardena AK. Open pancreatic necrosectomy in the multidisciplinary management of postinflammatory necrosis. Ann Surg. 2010;251(5):783–786.
- Connor S, Alexakis N, Raraty MG, Ghaneh P, Evans J, Hughes M, Garvey CJ, Sutton R, Neoptolemos JP. Early and late complications after pancreatic necrosectomy. Surgery. 2005;137(5):499–505.
- Parekh D. Laparoscopic-assisted pancreatic necrosectomy: a new surgical option for treatment of severe necrotizing pancreatitis. Arch Surg. 2006;141(9):895–902; discussion 902–893.
- Raraty MG, Halloran CM, Dodd S, et al. Minimal access retroperitoneal pancreatic necrosectomy: improvement in morbidity and mortality with a less invasive approach. Ann Surg. 2010;251(5):787–793.
- Adams DB, Harvey TS, Anderson MC. Percutaneous catheter drainage of infected pancreatic and peripancreatic fluid collections. Arch Surg. 1990;125(12):1554–1557.
- Freeny PC, Hauptmann E, Althaus SJ, Traverso LW, Sinanan M. Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. AJR Am J Roentgenol. 1998;170(4):969–975.
- Segal D, Mortele KJ, Banks PA, Silverman SG. Acute necrotizing pancreatitis: role of CT-guided percutaneous catheter drainage. Abdom Imaging. 2007;32(3):351–361.
- Rocha FG, Benoit E, Zinner MJ, et al. Impact of radiologic intervention on mortality in necrotizing pancreatitis: the role of organ failure. Arch Surg. 2009;144(3):261–265.
- Fernandez-del Castillo C, Rattner DW, Makary MA, Mostafavi A, McGrath D, Warshaw AL. Débridement and closed packing for the treatment of necrotizing pancreatitis. Ann Surg. 1998;228(5):676–684.
- Buchler MW, Gloor B, Muller CA, Friess H, Seiler CA, Uhl W. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232(5):619–626.
- Beger HG, Buchler M, Bittner R, Oettinger W, Block S, Nevalainen T. Necrosectomy and postoperative local lavage in patients with necrotizing pancreatitis: results of a prospective clinical trial. World J Surg. 1988;12(2):255–262.
- Howard TJ, Patel JB, Zyromski N, et al. Declining morbidity and mortality rates in the surgical management of pancreatic necrosis. J Gastrointest Surg. 2007;11(1):43–49.
- Tsiotos GG, Luque-de Leon E, Sarr MG. Long-term outcome of necrotizing pancreatitis treated by necrosectomy. Br J Surg. 1998;85(12):1650–1653.
- Parikh PY, Pitt HA, Kilbane M, Howard TJ, Nakeeb A, Schmidt CM, Lillemoe KD, Zyromski NJ. Pancreatic necrosectomy: North American mortality is much lower than expected. J Am Coll Surg. 2009;209(6):712–719.
- Carter CR, McKay CJ, Imrie CW. Percutaneous necrosectomy and sinus tract endoscopy in the management of infected pancreatic necrosis: an initial experience. Ann Surg. 2000;232(2):175–180.
- Horvath K, Brody F, Davis B, et al. Minimally invasive management of pancreatic disease: SAGES and SSAT pancreas symposium, Ft. Lauderdale, Florida, April 2005. Surg Endosc. 2007;21(3):367–372.
- Horvath KD, Kao LS, Ali A, Wherry KL, Pellegrini CA, Sinanan MN. Laparoscopic assisted percutaneous drainage of infected pancreatic necrosis. Surg Endosc. 2001;15(7):677–682.
- Fotoohi M, D'Agostino HB, Wollman B, Chon K, Shahrokni S. Persistent pancreatocutaneous fistula after percutaneous drainage of pancreatic fluid collections: role of cause and severity of pancreatitis. Radiology. 1999;213(2):573–578.
- Gagner M. Laparoscopic treatment of acute necrotizing pancreatitis. Semin Laparosc Surg. 1996;3(1):21–28.
- Zhu JF, Fan XH, Zhang XH. Laparoscopic treatment of severe acute pancreatitis. Surg Endosc. 2001;15(2):146–148.
- Cuschieri A. Pancreatic necrosis: pathogenesis and endoscopic management. Semin Laparosc Surg. 2002;9(1):54–63.
- Zhou ZG, Zheng YC, Shu Y, et al. Laparoscopic management of severe acute pancreatitis. Pancreas. 2003;27(3):e46–e50.
- van Brunschot S, van Grinsven J, Voermans RP, et al. Transluminal endoscopic step-up approach versus minimally invasive surgical step-up approach in patients with infected necrotising pancreatitis (TENSION trial): design and rationale of a randomised controlled multicenter trial [ISRCTN09186711]. BMC Gastroenterol. 2013;13:161.