Promotility Agents for the Treatment of Ileus in Adult Surgical Patients
Published 2019
Citation: J Trauma. 87(4):922-934, October 2019
Authors
Bugaev, Nikolay MD; Bhattacharya, Bishwajit MD; Chiu, William C. MD; Como, John J. MD, MPH; Cripps, Michael W. MD; Ferrada, Paula MD; Gelbard, Rondi B. MD; Gondek, Stephen MD, MPH; Kasotakis, George MD, MPH; Kim, Dennis MD; Mentzer, Caleb DO; Robinson, Bryce R. H. MD; Salcedo, Edgardo S. MD; Yeh, D. Dante MD
Author Information
From the Division of Trauma and Acute Care Surgery (N.B.), Tufts Medical Center, Tufts University School of Medicine. Boston, Massachusetts; Department of Surgery (B.B.), Yale School of Medicine, New Haven, Connecticut; Department of Surgery, University of Maryland School of Medicine, R Adams Cowley Shock Trauma Center, (W.C.C.), Baltimore, Maryland; Department of Surgery (J.J.C.), MetroHealth Medical Center, Cleveland, Ohio; Trauma Division of General and Acute Care Surgery, Department of Surgery, University of Texas Southwestern Medical Center (M.W.C.), Dallas, Texas; Virginia Commonwealth University (P.F.), Richmond, VA; Department of Surgery, Division of Trauma and Surgical Critical Care at Grady Memorial Hospital, Emory University School of Medicine (R.B.G.), Atlanta, Georgia; Division of Trauma and Surgical Critical Care (S.G.), Vanderbilt University Medical Center, Nashville, Tennessee; Division of Trauma and Critical Care Surgery, Department of Surgery (G.K.), Duke University School of Medicine, Durham, North Carolina; Division of Trauma/Acute Care Surgery/Surgical Critical Care (D.K.), LA County Harbor-UCLA Medical Center, Torrance, California; Division of Trauma, Critical Care, and Acute Care Surgery (C.M.), Spartanburg Medical Center, Spartanburg, South Carolina; Harborview Medical Center (B.R.H.R.), University of Washington, Seattle, Washington; Division of Trauma, Acute Care Surgery and Surgical Critical Care, Department of Surgery, University of California Davis (E.S.S.), Sacramento, California; and Ryder Trauma Center (D.D.Y.), University of Miami, Miami, Florida
Submitted: October 21, 2018, Revised: April 10, 2019, Accepted: May 5, 2019, Published online: May 24, 2019.
The article was presented as a podium presentation at the 31st Eastern Association for the Surgery of Trauma (EAST) Annual Scientific Assembly. January 11, 2018: Lake Buena Vista, Florida.
Address for reprints: Nikolay Bugaev, MD, Division of Trauma & Acute Care Surgery, Tufts Medical Center, Tufts University School of Medicine, Boston, MA 800 Washington St, 4488, Boston, MA 02111; email: nbugaev@tuftsmedicalcenter.org.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
Online date: May 29, 2019
BACKGROUND Ileus is a common challenge in adult surgical patients with estimated incidence to be 17% to 80%. The main mechanisms of the postoperative ileus pathophysiology are fluid overload, exogenous opioids, neurohormonal dysfunction, gastrointestinal stretch, and inflammation. Management includes addressing the underlying cause and supportive care. Multiple medical interventions have been proposed, but effectiveness is uncertain. A working group of the Eastern Association for the Surgery of Trauma aimed to evaluate the effectiveness of metoclopramide, erythromycin, and early enteral nutrition (EEN) on ileus in adult surgical patients and to develop recommendations applicable in a daily clinical practice.
METHODS Literature search identified 45 articles appropriate for inclusion. The Grading of Recommendations Assessment, Development and Evaluation methodology was applied to evaluate the effect of metoclopramide, erythromycin, and EEN on the resolution of ileus in adult surgical patients based on selected outcomes: return of normal bowel function, attainment of enteral feeding goal, and hospital length of stay. The recommendations were made based on the results of a systematic review, a meta-analysis, and evaluation of levels of evidence.
RESULTS The level of evidence for all PICOs was assessed as low. Neither metoclopramide nor erythromycin were effective in expediting the resolution of ileus. Analyses of 32 randomized controlled trials showed that EEN facilitates return of normal bowel function, achieving enteral nutrition goals, and reducing hospital length of stay.
CONCLUSION In patients who have undergone abdominal surgery, we strongly recommend EEN to expedite resolution of Ileus, but we cannot recommend for or against the use of either metoclopramide or erythromycin to hasten the resolution of ileus in these patients.
LEVEL OF EVIDENCE Type of Study Therapeutic, level II.
Ileus, a type of abnormal gastrointestinal motility, is a common phenomenon in patients who have undergone abdominal surgery. Clinical symptoms of this condition include nausea, vomiting, and absence of flatus, and/or bowel movements. The true incidence is unknown but is estimated to be 17% to 80%.[1][2]
The main mechanisms of the postoperative ileus pathophysiology are fluid overload, neurohormonal dysfunction, gastrointestinal stretch and inflammation.[2] The etiology of ileus is multifactorial and includes abdominal, thoracic, and spine operations, sepsis, and disturbances of fluid-electrolyte balance.[3] A type of the abdominal surgery also plays a role in the development of the ileus with abdominal hysterectomy and appendectomy having the lowest rate, 4.1% to 6.%, while small bowel resection has the incidence of ileus as high as 19.2%.[4] Multiple medications were reported to be associated with the development of ileus such as calcium channel blockers, clonidine, antineoplastic agents, antidiarrheal/antispasmodic, phenothiazine antiemetics, oral iron preparations, selective serotonin reuptake inhibitor antidepressants, tricyclic antidepressants, antipsychotics, Parkinson disease medications, first generation of H1 antihistamines, muscle relaxants, atropine products.[5]
Ileus has been shown to be associated with increased risks of aspiration, pneumonia, decreased rate of enteral feeding, increased hospital length of stay (LOS), and mortality.[6–8] An analysis of the Premier's Perspective Comparative Database, a repository of US hospital administrative data, showed that the mean LOS of patients with postoperative ileus versus those without one was 11.5 days versus 5.5 days and the mean cost of the inpatient stay was US $18,877 vs. US $9,640, respectively.[4]
The management of ileus is directed toward correction of the underlying cause, fluid-electrolyte balance, and avoidance of medications associated with ileus. The Cochrane review in 2008 summarized comparison effect of 10 systematic prokinetic agents for adynamic ileus.[9] Overall, all included studies had a poor methodological quality. Usage of alvimopan was supported by six trials. Erythromycin did not show a positive effect on the ileus. Effect of Cholecystokinin-like drugs, cisapride, dopamine-antagonists (domperidone), propranolol, vasopressin, intravenous lidocaine and neostigmine was found either inconsistent or required more evidence on clinically relevant outcomes. The use of several other prokinetic agents has been proposed to hasten the resolution of ileus, including metoclopramide, naloxone, tegaserod, mitemcinal, ghrelin, prucalopride, and dexloxiglumide.[10] Few additional agents, which are not available in the United States, are considered to have a prokinetic effect: cisapride, levosulpiride, tegaserod, mosapride citrate, itopride hydrochloride, renzapride. However, their effectiveness is unclear.
The goal of this review was to evaluate the existing evidence and create recommendations regarding the routine use of metoclopramide, erythromycin, and early enteral nutrition (EEN) in surgical patients with ileus.
Objectives
The objective of this review was to evaluate the effect of metoclopramide, erythromycin, and early enteral feeding on the resolution of ileus in adult surgical patients, using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.[11] The GRADE is a methodology that, through standardized approach, rates a body of evidence and makes recommendations to address specific clinical questions. The Eastern Association for the Surgery of Trauma (EAST) Ileus practice management group was created on a voluntary base from EAST members. The working group discussed different agents and voted to evaluate three of the most commonly used interventions to treat ileus include metoclopramide, erythromycin, and early enteral feeding. The group aimed to perform a systematic review and create practical management guidelines regarding usage of metoclopramide, erythromycin and EEN to hasten resolution of ileus in adult surgical patients.
Adult surgical patients were defined as those patients who underwent either elective or emergent abdominal surgery.
Through an iterative voting process, the EAST Ileus practice manage management workgroup developed the following PICO questions (Population, Intervention, Comparison, Outcomes):
PICO 1
In adult surgical patients (P) with ileus, should treatment with metoclopramide be instituted (I), versus usual care without metoclopramide (C), to accelerate return of normal bowel function and attainment of enteral feeding goal, and to decrease hospital LOS?
PICO 2
In adult surgical patients (P) with ileus, should treatment with erythromycin be instituted (I), versus usual care without erythromycin (C), to accelerate return of normal bowel function and attainment of enteral feeding goal, and to decrease hospital LOS?
PICO 3
In adult surgical patients (P) with ileus, should early enteral feeding (defined as enteral nutrition that was started during the first 48 hours after the surgery) be instituted (I), compared with usual care (C), to accelerate return of normal bowel function and attainment of enteral feeding goal, and to decrease hospital LOS?
Outcome Measure Type
The members of the working group proposed outcomes related to resolution of ileus. All outcomes were independently rated by each group member on a scale from 1 to 9 and the median score for each outcome was calculated and assigned as the final score.
Outcomes scored between 7 and 9 were considered critical and included: resolution of ileus, time to first flatus, time to first bowel movement, duration of nasogastric tube (NGT), NGT reinsertion rate, attainment of enteral feeding goal, and hospital LOS. The working group decided to evaluate the resolution of ileus utilizing the following outcomes: return of normal bowel function, attainment of enteral feeding goal, and hospital LOS.
The outcome “return of normal bowel function” was based on: time to first flatus, time to first bowel movement, duration of NGT and NGT reinsertion rate.
The original outcome voting also contained nutritional adequacy, tolerance of solid food, tolerance of enteral nutrition, and time to achieve goal enteral nutrition. Given the significant heterogeneity in the measurement of goal enteral feeding among studies, the above outcomes were combined into the “attainment of enteral feeding goal” outcome, which was deemed as a critical outcome.
Identification of References
A medical librarian performed a search of citations in the following databases: PubMed, CINAHIL, Embase, Cochrane Library, Web of Science, PROSPERO, RefWorks, and Scopus. The search was performed using the following MeSH terms: “Ileus,” “Metoclopramide”, “Erythromycin”, “Metoclopramide”, “Reglan”, “Metozolv”, “Maxolon,” “Rimetin,” “Rimperan”, “Cerucal”, “Erythromycin, “Trophic”, “Early enteral”,” Early enteral feeding,” “Early enteral hypocaloric,” “Gut priming,” “Minimal enteral,” “Minimal enteral feeding,” “Minimal enteral feeds,” “Minimal enteral intake,” and “Minimal enteral nutrition.”
Original clinical retrospective studies, prospective observational studies, and randomized controlled trials (RCT) in adults (age, ≥18 years) were considered for inclusion. Review articles, meta-analyses, case reports, case series without a comparison group, manuscripts that evaluated colonic pseudo-obstruction, and non-English language publications were excluded.
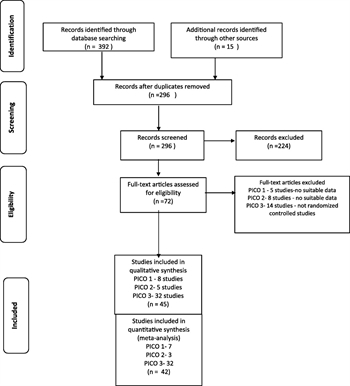
Two members of the working group independently screened titles and abstracts of the selected references removing obviously irrelevant reports. Next, full-text articles were independently screened by two separate working group members, aiming to include reports that were in compliance with a priory chosen eligibility criteria. Selected studies were included for final data extraction and analysis. At each stage of the screening process, disagreements between the reviewers were adjudicated by discussion and consensus among the individuals. When consensus was not reached, a third reviewer was involved as an arbitrator (Fig. 1).
Data Extraction and Methodology
A total of 45 studies were included.[12–56] Data extraction was performed by two independent team members for each of the selected studies and entered into a Microsoft Excel 2010 (Redmond, WA) spreadsheet. The meta-analysis and creation of forest plots was performed using Review Manager (RevMan) (Version 5.3; Cochrane Collaboration, Oxford). Dichotomous outcomes were reported as risk ratio (RR), and continuous variables were reported as standardized mean difference (SMD). Confidence interval (CI) of 95% was presented with RR and SMD. For studies that reported continuous variables as median and range[15][18][20][23][24][41][46][47][49][50][54] means and standard deviations were estimated in order to be able to perform the meta-analysis.[57]
All time-related outcomes were presented in days.
Risk of Bias
Risk of bias of the included RCT was assessed in six domains: sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and “other issues” (Supplemental Digital Content 1–3, Figs. 1–3, http://links.lww.com/TA/B414, http://links.lww.com/TA/B415, http://links.lww.com/TA/B416).
Grading the Evidence
The available evidence was assessed according to the GRADE methodology as high, moderate, low, or very low quality. The quality of evidence was downgraded for inconsistency, indirectness, and imprecision.
Results for the Use of Metoclopramide (PICO 1)
In adult surgical patients (P) with ileus, should treatment with metoclopramide be instituted (I), versus usual care without metoclopramide (C), to accelerate return of normal bowel function and attainment of enteral feeding goal, and to decrease hospital LOS?
Qualitative Analysis
Our search yielded a total of eight studies: three RCT,[15][17][18] three prospective with control groups,[12][13][16] and two retrospective with control groups.[14][19] The included studies contained 543 patients in the intervention group and 599 in control groups. The selected studies included a heterogeneous patient population: patients requiring intensive care unit after abdominal surgeries,[10] and non-intensive care unit surgical patients who underwent abdominal operations for variety of indications.[12][14–19] Overall, included studies were heterogeneous in terms of inclusion/exclusion criteria, the dose of the intervention, and how the clinical effect of metoclopramide on the ileus was reported. Studies where metoclopramide was combined with another agent were excluded.
In only one study was metoclopramide compared with placebo.[15] In seven studies, metoclopramide was compared with no intervention: no placebo or other medication.[12–14][16–19] In one study, metoclopramide was a part of fast-track program[19]; the control arm also included the fast-track program but without metoclopramide.
Overall, five studies concluded that metoclopramide had a beneficial effect on ileus.[12][14][16][18][19] The positive metoclopramide effect was based on different criteria: return of normal bowel function: time to first bowel movement,[18] duration of NGT,[14][16] NGT reinsertion rate[12]; attainment of enteral feeding goal,[12][14][16][18] and LOS.[14] Pruthi et al.[19] concluded a positive effect of metoclopramide based on a decreased rate of nausea and vomiting, but there was no effect on the PMG-selected outcomes (return of normal bowel function and LOS).
In the studies where no positive effect was found,[13][15][17] this conclusion was based on lack of return of normal bowel function: time to first flatus,[15][17] time to first bowel movement,[13][17] NGT reinsertion rate,[17] attainment of goal enteral feeding,[13][15][17] and hospital LOS.[13]
The postoperative complication rate in the metoclopramide groups was not significantly higher in the studies that reported them.[16][19] No adverse events related to the usage of metoclopramide were reported.
Quantitative Analysis (Meta-analysis)
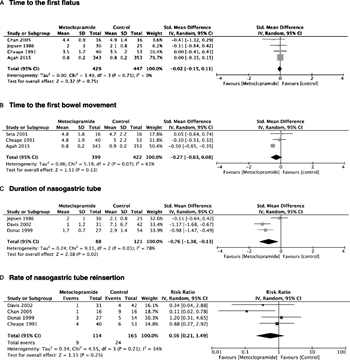
Figure 2. Metoclopramide. A, Time to the first flatus. B, Time to the first bowel movement. C, Duration of NGT. D, Rate of NGT reinsertion. E, Attainment of goal enteral feeding. F, Hospital LOS.
Seven out of the eight studies were suitable for meta-analysis. There was a beneficial effect of metoclopramide on the duration of NGT (SMD, −0.76; 95% CI, −1.38 to −0.13), the rate of NGT reinsertion (RR, 0.56; 95% CI, 0.21–1.49), and hospital LOS (SMD, −0.42; 95% CI, −0.72 to −0.12) (Fig. 2C, 2D, 2F).
With regard to the time to first flatus, time to first bowel movement, and the attainment of enteral feeding goals, the effect of metoclopramide was uncertain (Fig. 2A, 2B, 2E).
Grading the Evidence
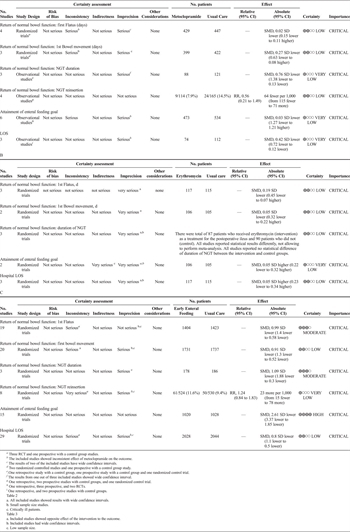
The evidence was assessed applying the GRADE framework (Table 1). First, the level of evidence was decreased for all outcomes due to the inclusion of retrospective and prospective observational studies. We also downgraded the level of evidence for inconsistency due to inconsistent effect of metoclopramide on the time to first flatus, time to first bowel movement, and attainment of goal enteral feeding. Since wide confidence intervals were reported for the time to first flatus, time to first bowel movement, and attainment of goal enteral feeding, the level of evidence was further decreased for imprecision.
The bias assessment revealed that 33% of included RC had a lack of blinding of either participant or the research staff to the studies' group assignments (performance bias). Also sequence generation and allocation concealment (selection bias) was not clearly described in all included studies (Supplemental Digital Content 1, Fig. 1, http://links.lww.com/TA/B414). Because of these factors, the quality of evidence was deemed to be low.
Recommendations for the Use of Metoclopramide (PICO 1)
Based on the analysis of included studies, the effect of metoclopramide on the selected outcomes, and the low level of evidence we cannot make a recommendation for or against the use of metoclopramide in adult surgical patients to hasten ileus resolution. Although the usage of metoclopramide was not associated with adverse outcomes, the effect on ileus resolution and attainment of enteral nutrition goal was inconsistent.
Results for the Use of Erythromycin (PICO 2)
In adult surgical patients (P) with ileus, should treatment with erythromycin be instituted (I), versus usual care without erythromycin (C), to accelerate return of normal bowel function and attainment of enteral feeding goal, and to decrease hospital LOS?
Qualitative Analysis
There were five studies selected for the analysis for PICO 2. Four studies were RCT comparing erythromycin to placebo.[20][21][23][24] We also included one prospective study with a control group[22] in which patients received erythromycin versus usual care for ileus. There were a total of 128 patients treated with erythromycin and 140 patients in the control groups.
The selected studies included patients who underwent abdominal operations for a variety of reasons.[20–24] Overall, included studies were heterogeneous in terms of inclusion/exclusion criteria and dose of intervention (from a few day course until oral diet was tolerated or a fixed number of days, 2 days to 7 days.[20–24] Also, the studies reported the effect of erythromycin on ileus using different parameters.
No beneficial effect of erythromycin on the resolution of ileus was found in any of the included studies. These conclusions were made based on the following: no effect on time to first flatus,[20–24] time to first bowel movement,[20][22][24] duration of NGT,[20][21][24] and tolerance of enteral diet.[20][23][24]
The postoperative complication rate in the erythromycin groups was not significantly higher in the studies that reported them.[20][21][23][24] An erythromycin-related skin rash was reported in only in four out of 106 patients.[23][24]
Quantitative Analysis
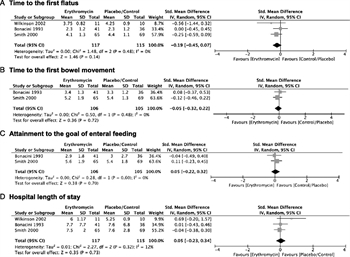
Figure 3. Erythromycin. A, Time to the first flatus. B, Time to the first bowel movement. C, Attainment to the goal of enteral feeding. D. Hospital LOS.
Three[21][23][24] of five studies were suitable for meta-analysis (Fig. 3). The meta-analysis failed to show a beneficial effect of erythromycin on time to first flatus (SMD, −0.19; 95% CI, −0.45 to 0.07), time to first bowel movement (SMD, −0.05; 95% CI, −0.32 to 0.22), attainment of enteral feeding goal (SMD, 0.05; 95% CI, −0.22 to 0.32), or hospital LOS (SMD, 0.05; 95% CI, −0.23 to 0.34).
Grading the Evidence
The evidence was assessed applying the GRADE framework (Table 2). The level of evidence was decreased for imprecision when results were reported with wide confidence intervals: time to first flatus, time to first bowel movement, and hospital LOS. The level of evidence was downgraded for imprecision, as all included studies had low sample sizes.
The bias assessment revealed that 75% of included RC did not describe either sequence generation or allocation concealment (selection bias). About 50% of the included studies had a lack of blinding of outcome assessment (detection bias) (Supplemental Digital Content 2, Figure 2, http://links.lww.com/TA/B415). The overall quality of evidence was assessed as low.
Recommendations for the Use of Erythromycin (PICO 2)
Based on the analyses of included studies, the effect of erythromycin on the selected outcomes, and the level of evidence, we cannot make a recommendation for or against the use of erythromycin in adult surgical patients to hasten ileus resolution.
Results for the Use of EEN (PICO 3)
In adult surgical patients (P) with ileus, should early enteral feeding (defined as enteral nutrition that was started during the first 48 hours after the surgery) be instituted (I), compared to usual care (C), to accelerate return of normal bowel function and attainment of enteral feeding goals, and to decrease hospital LOS?
Qualitative Analysis
Given the large number of publications on this subject, we included only RCT. There were 32 RCT studies selected for the analysis for PICO 3.[25–56] There were a total of 2,398 patients treated with EEN, and there were 2,242 patients in the control groups.
The selected studies included patients who underwent elective gastrointestinal surgeries,[25–33] elective colorectal surgery,[34–47] vascular surgery,[35][43] emergent abdominal surgery,[48] and gynecologic surgery.[49–56] Overall, included studies were heterogeneous in terms of inclusion/exclusion criteria and in the way EEN was delivered (oral diet or tube feeding). In all included studies, EEN was started during the first 24 hours to 48 hours after admission to the hospital or after the surgical procedure. In the control groups, enteral nutrition was started after ileus resolution[25][27–30][32][33][35–56] or parenteral nutrition was started on the same day of the surgery.[26][31][34]
Overall EEN was found to be safe and well tolerated,[25][27–29][32–34][36–41][43–54][56] resulted in faster return of normal bowel function,[25][27][30][34][40][42][47][51] and improved nutritional status.[26][29][32][43] Three studies reported no beneficial effect of EEN on the resolution of ileus.[35][49][53] The overall the rate of adverse effects related to EEN, such as nausea, vomiting, wound infections, anastomotic leaks and pulmonary complications, did not differ between EEN and comparison groups.
Quantitative Analysis
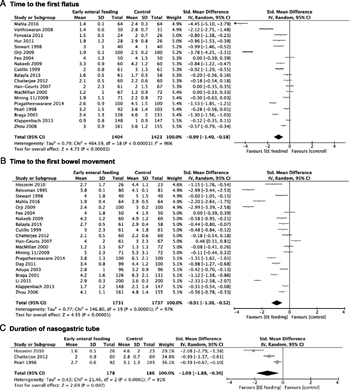
Figure 4. Early enteral nutrition. A, Time to the first flatus. B, Time to the first bowel movement. C, Duration of NGT. D, Rate of NGT reinsertion. E, Attainment to the goal of enteral feeding. F, Hospital LOS.
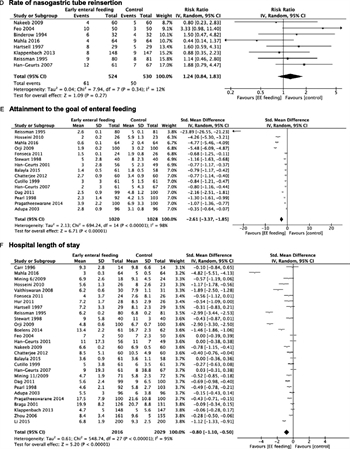
Figure 4. Early enteral nutrition. A, Time to the first flatus. B, Time to the first bowel movement. C, Duration of NGT. D, Rate of NGT reinsertion. E, Attainment to the goal of enteral feeding. F, Hospital LOS.
All studies were included in the meta-analysis. EEN was associated with faster return of normal bowel function, defined as: time to first flatus (SMD, −0.99; 95% CI, −1.40 to −0.58), time to first bowel movement (SMD, −0.91; 95% CI, −1.30 to −0.52), duration of NGT (SMD, −1.09; 95% CI, −1.88 to −0.30), NGT reinsertion rate (RR, 1.24; 95% CI, 0.84–1.83); attainment of enteral feeding goal (SMD, −2.61; 95% CI, −3.37 to −1.85), and hospital LOS (SMD, −0.80; 95% CI, −1.10 to −0.50) (Fig. 4).
Grading the Evidence
The evidence was assessed applying the GRADE framework (Table 3). The level of evidence was decreased for imprecision when results were reported with wide confidence intervals in NGT reinsertion rate.
The bias assessment revealed a significant lack of blinding of either the participant or the research staff to the studies' group assignments (performance bias). Also sequence generation and allocation concealment (selection bias) was not clearly described in more than half of the included studies (Supplemental Digital Content 3, Figure 3, http://links.lww.com/TA/B416). Overall the level of evidence for PICO 3 was assessed as low.
Recommendations for the Use of EEN (PICO 3)
Based on the analysis of included studies, the effect of EEN on the selected outcomes, and the quality of the evidence, we strongly recommend using EEN in adult surgical patients to hasten ileus resolution.
Using these Guidelines in Clinical Practice
This systematic review evaluated the effects of metoclopramide, erythromycin, and EEN on the resolution of ileus in surgical patients.
Early enteral nutrition was found to be effective and safe in facilitating resolution of ileus. The included studies defined EEN as enteral nutrition in any dosage that was initiated within 24 hours to 48 hours after the operation. EEN reduced time to return of normal bowel function, and shortened hospital LOS. The adverse events attributed to EEN were minimal and did not affect clinically relevant patient outcomes. Although the level of evidence supporting our conclusion was low, the overwhelming beneficial effect of EEN on the selected outcomes, the large number of included RCT and patients, and the safety of EEN allowed us to make a strong recommendation to use EEN to accelerate resolution of ileus.
The evidence to support usage of metoclopramide or erythromycin in surgical patients was poor. None of the selected outcomes (return of normal bowel function, attainment of enteral feeding goal, and hospital LOS) were positively affected by either metoclopramide or erythromycin. Only a few studies reported complications in either the metoclopramide or erythromycin groups; the incidence of these events was low and did not affect patient outcomes. No metoclopramide-related adverse events were reported. Only a few episodes of skin rash were described in the erythromycin groups. Based on the analysis of included studies, the effect of either metoclopramide or erythromycin on the selected outcomes, and low level of evidence, we could not make recommendations for or against the use of either metoclopramide or erythromycin in surgical patients to hasten the resolution of ileus.
Conclusion
For the use of metoclopramide in adult surgical patients to hasten ileus resolution, we cannot recommend for or against. We cannot recommend for or against the use of erythromycin in adult surgical patients to hasten ileus resolution. We strongly recommend using EEN in adult surgical patients to hasten ileus resolution (Table 4).
Authorship
N.B. participated in the study design, literature search, data collection, data analysis, data interpretation, drafting the article. B.B. participated in the study design, data collection, data interpretation, and critical revisions. W.C. participated in the study design, data collection, data interpretation, and critical revisions. J.C. participated in the study design, data collection, data interpretation, and critical revisions. M.C. participated in the study design, data collection, data interpretation, and critical revisions. P.F. participated in the study design, data collection, data interpretation, and critical revisions. R.G. participated in the study design, data collection, data interpretation, and critical revisions. S.G. participated in the study design, data collection, data interpretation, and critical revisions. G.K. participated in the study design, data collection, data interpretation, and critical revisions. D.K. participated in the study design, data collection, data interpretation, and critical revisions. C.M. participated in the study design, data collection, data interpretation, and critical revisions. B.R. participated in the study design, data collection, data interpretation, and critical revisions. E.S. participated in the study design, data collection, data interpretation, and critical revisions, and D.D.Y. participated in the study idea, study design, literature search, data collection, data analysis, data interpretation, and critical revisions.
Disclosure
The authors declare no conflicts of interest.
References
- Caddell KA, Martindale R, McClave SA, Miller K. Can the intestinal dysmotility of critical illness be differentiated from postoperative ileus? Curr Gastroenterol Rep. 2011;13(4):358–367.
- Bragg D, El-Sharkawy AM, Psaltis E, Maxwell-Armstrong CA, Lobo DN. Postoperative ileus: recent developments in pathophysiology and management. Clin Nutr. 2015;34(3):367–376.
- Taylor RW. Gut motility issues in critical illness. Crit Care Clin. 2016;32(2):191–201.
- Goldstein JL, Matuszewski KA, Delaney C, Senagore A, Chiao E, Shah M, Meyer K, Bramley T. Inpatient economic burden of postoperative ileus associated with abdominal surgery in the United States. P&T. 2007;32:82–90.
- UpToDate. Nonsurgical causes of ileus. https://www.uptodate.com/contents/image?topicKey=SURG%2F8042&view=machineLearning&search=ileus%20paralytic§ionRank=1&imageKey=SURG%2F96322&rank=1~150&source=machineLearning&sp=1, Accessed March 20, 2019.
- Reintam Blaser A, Malbrain ML, Stakopf J, Fruhwald S, Jakob SM, De Waele J, Braun JP, Poeze M, Spies C. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM working group on abdominal problems. Intensive Care Med. 2012;38:384–394.
- Rombeau JL, Takala J. Summary of round table conference: gut dysfunction in critical illness. Intensive Care Med. 1997;23:476–479.
- Hill LT. Gut dysfunction in the critically ill—mechanisms and clinical implications. S Afr J Crit Care. 2013;29:11–15.
- Traut U, Brügger L, Kunz R, Pauli-Magnus C, Haug K, Bucher HC, Koller MT. Systemic prokinetic pharmacologic treatment for postoperative adynamic ileus following abdominal surgery in adults. Cochrane Database Syst Rev. 2008;1:CD004930.
- Ladopoulos T, Giannaki M, Alexopoulou C, Proklou A, Pediaditis E, Kondili E. Gastrointestinal dysmotility in critically ill patients. Ann Gastroenterol. 2018;31(3):273–281.
- Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, Dahm P, Eastern Association for the Surgery of Trauma Practice Management Guidelines Ad Hoc Committee. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S283–S287.
- Chan DC, Liu YC, Chen CJ, Yu JC, Chu HC, Chen FC, Chen TW, Hsieh HF, Chang TM, Shen KL. Preventing prolonged post-operative ileus in gastric cancer patients undergoing gastrectomy and intra-peritoneal chemotherapy. World J Gastroenterol. 2005;11(31):4776–4781.
- Seta ML, Kale-Pradhan PB. Efficacy of metoclopramide in postoperative ileus after exploratory laparotomy. Pharmacotherapy. 2001;21(10):1181–1186.
- Davis JW, Pisters LL, Doviak MJ, Donat SM. Early nasogastric tube removal combined with metoclopramide after post chemotherapy retroperitoneal lymph node dissection for metastatic testicular non seminomatous germ cell tumor. Urology. 2002;59(4):579–583.
- Jepsen S, Klaerke A, Nielsen PH, Simonsen O. Negative effect of metoclopramide in postoperative adynamic ileus. A prospective, randomized, double blind study. Br J Surg. 1986;73(4):290–291.
- Donat SM, Slaton JW, Pisters LL, Swanson DA. Early nasogastric tube removal combined with metoclopramide after radical cystectomy and urinary diversion. J Urol. 1999;162(5):1599–1602.
- Cheape JD, Wexner SD, James K, Jagelman DG. Does metoclopramide reduce the length of ileus after colorectal surgery? A prospective randomized trial. Dis Colon Rectum. 1991;34(6):437–441.
- Agah J, Baghani R, Rakhshani MH. Metoclopramide role in preventing ileus after cesarean, a clinical trial. Rad A. Eur J Clin Pharmacol. 2015;71(6):657–662.
- Pruthi RS, Nielsen M, Smith A, Nix J, Schultz H, Wallen EM. Fast track program in patients undergoing radical cystectomy: results in 362 consecutive patients. J Am Coll Surg. 2010;210(1):93–99.
- Lightfoot AJ, Eno M, Kreder KJ, O'Donnell MA, Rao SS, Williams RD. Treatment of postoperative ileus after bowel surgery with low-dose intravenous erythromycin. Urology. 2007;69(4):611–615.
- Wilkinson NW, Gustafson RJ, Frizzi JD. The effect of erythromycin on bile excretion and proximal small bowel motility following divided gastric bypass surgery: a prospective randomized placebo-controlled trial. Obes Surg. 2002;12(6):765–772.
- Lee AL, Kim CB. The effect of erythromycin on gastrointestinal motility in subtotal gastrectomized patients. J Korean Surg Soc. 2012;82(3):149–155.
- Bonacini M, Quiason S, Reynolds M, Gaddis M, Pemberton B, Smith O. Effect of intravenous erythromycin on postoperative ileus. Am J Gastroenterol. 1993;88(2):208–211.
- Smith AJ, Nissan A, Lanouette NM, Shi W, Guillem JG, Wong WD, Thaler H, Cohen AM. Prokinetic effect of erythromycin after colorectal surgery: randomized, placebo-controlled, double-blind study. Dis Colon Rectum. 2000;43(3):333–337.
- Chatterjee S, Bala SK, Chakraborty P, Dey R, Sinha S, Ray R, Rahed A. A comparative study between early enteral feeding (within 24 hours) versus conventional enteral feeding after enteric anastomosis. Bangladesh J Med Sci. 2012;11(4):273–282.
- Li B, Liu HY, Guo SH, Sun P, Gong FM, Jia BQ. The postoperative clinical outcomes and safety of early enteral nutrition in operated gastric cancer patients. J BUON. 2015;20(2):468–472.
- Mahla V, Khan S, Ahmad R, Jenaw RK. Early feeding after loop ileostomy reversal: a prospective study. Formosan J Surg. 2016;49(5):178–182.
- Hosseini SN, Mousavinasab SN, Rahmanpour H, Sotodeh S. Comparing early oral feeding with traditional oral feeding in upper gastrointestinal surgery. Turk J Gastroenterol. 2010;21(2):119–124.
- Vaithiswaran V, Srinivasan K, Kadambari D. Effect of early enteral feeding after upper gastrointestinal surgery. Trop Gastroenterol. 2008;29(2):91–94.
- Barlow R, Price P, Reid TD, Hunt S, Clark GW, Havard TJ, Puntis MC, Lewis WG. Prospective multicenter randomized controlled trial of early enteral nutrition for patients undergoing major upper gastrointestinal surgical resection. Clin Nutr. 2011;30(5):560–566.
- Braga M, Gianotti L, Gentilini O, Parisi V, Salis C, Di Carlo V. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit Care Med. 2001;29(2):242–248.
- Carr CS, Ling KD, Boulos P, Singer M. Randomised trial of safety and efficacy of immediate postoperative enteral feeding in patients undergoing gastrointestinal resection. BMJ. 1996;312(7035):869–871.
- Pragatheeswarane M, Muthukumarassamy R, Kadambari D, Kate V. Early oral feeding vs. traditional feeding in patients undergoing elective open bowel surgery-a randomized controlled trial. J Gastrointest Surg. 2014;18(5):1017–1023.
- Boelens PG, Heesakkers FF, Luyer MD, van Barneveld KW, de Hingh IH, Nieuwenhuijzen GA, Roos AN, Rutten HJ. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: prospective, randomized, controlled trial. Ann Surg. 2014;259(4):649–655.
- Han-Geurts IJ, Hop WC, Kok NF, Lim A, Brouwer KJ, Jeekel J. Randomized clinical trial of the impact of early enteral feeding on postoperative ileus and recovery. Br J Surg. 2007;94(5):555–561.
- Reissman P, Teoh TA, Cohen SM, Weiss EG, Nogueras JJ, Wexner SD. Is early oral feeding safe after elective colorectal surgery? A prospective randomized trial. Ann Surg. 1995;222(1):73–77.
- Hur H, Kim SG, Shim JH, Song KY, Kim W, Park CH, Jeon HM. Effect of early oral feeding after gastric cancer surgery: a result of randomized clinical trial. Surgery. 2011;149(4):561–568.
- Zhou T, Wu XT, Zhou YJ, Huang X, Fan W, Li YC. Early removing gastrointestinal decompression and early oral feeding improve patients' rehabilitation after colorectostomy. World J Gastroenterol. 2006;12(15):2459–2463.
- Binderow SR, Cohen SM, Wexner SD, Nogueras JJ. Must early postoperative oral intake be limited to laparoscopy? Dis Colon Rectum. 1994;37(6):584–589.
- Dag A, Colak T, Turkmenoglu O, Gundogdu R, Aydin S. A randomized controlled trial evaluating early versus traditional oral feeding after colorectal surgery. Clinics (Sao Paulo). 2011;66(12):2001–2005.
- Feo CV, Romanini B, Sortini D, Ragazzi R, Zamboni P, Pansini GC, Liboni A. Early oral feeding after colorectal resection: a randomized controlled study. ANZ J Surg. 2004;74(5):298–301.
- Da Fonseca LM, Profeta da Luz MM, Lacerda-Filho A, Correia MI, Gomes da Silva R. A simplified rehabilitation program for patients undergoing elective colonic surgery–randomized controlled clinical trial. Int J Colorectal Dis. 2011;26(5):609–616.
- Han-Geurts IJ, Jeekel J, Tilanus HW, Brouwer KJ. Randomized clinical trial of patient-controlled versus fixed regimen feeding after elective abdominal surgery. Br J Surg. 2001;88(12):1578–1582.
- Hartsell PA, Frazee RC, Harrison JB, Smith RW. Early postoperative feeding after elective colorectal surgery. Arch Surg. 1997;132(5):518–520; discussion 520-1.
- El Nakeeb A, Fikry A, El Metwally T, Fouda E, Youssef M, Ghazy H, Badr S, Khafagy W, Farid M. Early oral feeding in patients undergoing elective colonic anastomosis. Int J Surg. 2009;7(3):206–209.
- Ortiz H, Armendariz P, Yarnoz C. Is early postoperative feeding feasible in elective colon and rectal surgery? Int J Colorectal Dis. 1996;11(3):119–121.
- Stewart BT, Woods RJ, Collopy BT, Fink RJ, Mackay JR, Keck JO. Early feeding after elective open colorectal resections: a prospective randomized trial. Aust N Z J Surg. 1998;68(2):125–128.
- Klappenbach RF, Yazyi FJ, Alonso Quintas F, Horna ME, Alvarez Rodríguez J, Oría A. Early oral feeding versus traditional postoperative care after abdominal emergency surgery: a randomized controlled trial. World J Surg. 2013;37(10):2293–2299.
- MacMillan SL, Kammerer-Doak D, Rogers RG, Parker KM. Early feeding and the incidence of gastrointestinal symptoms after major gynecologic surgery. Obstet Gynecol. 2000;96(4):604–608.
- Pearl ML, Valea FA, Fischer M, Mahler L, Chalas E. A randomized controlled trial of early postoperative feeding in gynecologic oncology patients undergoing intra-abdominal surgery. Obstet Gynecol. 1998;92(1):94–97.
- Cutillo G, Maneschi F, Franchi M, Giannice R, Scambia G, Benedetti-Panici P. Early feeding compared with nasogastric decompression after major oncologic gynecologic surgery: a randomized study. Obstet Gynecol. 1999;93(1):41–45.
- Adupa D, Wandabwa J, Kiondo P. A randomised controlled trial of early initiation of oral feeding after caesarean delivery in Mulago Hospital. East Afr Med J. 2003;80(7):345–350.
- Balayla J, Bujold E, Lapensée L, Mayrand MH, Sansregret A. Early versus delayed postoperative feeding after major gynaecological surgery and its effects on clinical outcomes, patient satisfaction, and length of stay: a randomized controlled trial. J Obstet Gynaecol Can. 2015;37(12):1079–1085.
- Minig L, Biffi R, Zanagnolo V, et al. Early oral versus “traditional” postoperative feeding in gynecologic oncology patients undergoing intestinal resection: a randomized controlled trial. Ann Surg Oncol. 2009;16(6):1660–1668.
- Minig L, Biffi R, Zanagnolo V, et al. Reduction of postoperative complication rate with the use of early oral feeding in gynecologic oncologic patients undergoing a major surgery: a randomized controlled trial. Ann Surg Oncol. 2009;16(11):3101–3110.
- Orji EO, Olabode TO, Kuti O, Ogunniyi SO. A randomised controlled trial of early initiation of oral feeding after cesarean section. J Matern Fetal Neonatal Med. 2009;22(1):65–71.
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Keywords:
Ileus; metoclopramide; erythromycin; early enteral nutrition