Timing of Operative Debridement for Necrotizing Soft Tissue
Published 2018
Citation: J Trauma. 85(1):208-214, July 2018
Authors
Gelbard, Rondi B., MD; Ferrada, Paula, MD; Yeh, D. Dante, MD; Williams, Brian H., MD; Loor, Michele, MD; Yon, James, MD; Mentzer, Caleb, DO; Khwaja, Kosar, MD, MBA, MSc; Khan, Mansoor A., MBBS, PhD; Kohli, Anirudh, MD; Bulger, Eileen M., MD; Robinson, Bryce R.H., MD
Author Information
From the Division of Trauma and Surgical Critical Care at Grady Memorial Hospital, Department of Surgery, Emory University School of Medicine (R.B.G.), Atlanta, Georgia; Division of Acute Care Surgery, Department of Surgery, Virginia Commonwealth University (P.F.), Richmond, Virginia; Division of Trauma and Critical Care, Department of Surgery, University of Miami (D.D.Y., C.M.), Miami, Florida; Department of Surgery, UT Southwestern Medical Center (B.H.W.), Dallas, Texas; Division of Critical Care and Acute Care Surgery, Department of Surgery, University of Minnesota (M.L.), Minneapolis, Minnesota; Department of Surgery, Swedish Medical Center (J.Y.), Englewood, Colorado; Department of Surgery and Critical Care Medicine, McGill University Health Centre (K.K.), Montreal, Canada; St. Mary’s Hospital Major Trauma Center, Imperial College Healthcare (M.K.), London, United Kingdom; Division of Acute Care Surgery, Department of Surgery, Thomas Jefferson University Hospital (A.K.), Philadelphia, Pennsylvania; and Division of Trauma, Burn and Critical Care Surgery, Department of Surgery, Harborview Medical Center (E.M.B., B.R.H.R.), University of Washington, Seattle, Washington.
Submitted: October 4, 2017, Revised: January 18, 2018, Accepted: January 31, 2018, Published online: February 27, 2018.
Presented at: The Eastern Association for the Surgery of Trauma 30th Annual Scientific Assembly, January 10–14 2017, Hollywood, Florida.
Address for reprints: Rondi B. Gelbard, MD, Emory University School of Medicine, 69 Jesse Hill Jr. Drive, SE, Glenn Memorial Building, Room 315, Atlanta, Georgia 30303; email: rgelbar@emory.edu.
Abstract
BACKGROUND Necrotizing soft tissue infections (NSTI) are rare, life-threatening, soft-tissue infections characterized by rapidly spreading inflammation and necrosis of the skin, subcutaneous fat, and fascia. While it is widely accepted that delay in surgical debridement contributes to increased mortality, there are currently no practice management guidelines regarding the optimal timing of surgical management of this condition. Although debridement within 24 hours of diagnosis is generally recommended, the time ranges from 3 hours to 36 hours in the existing literature. Therefore, the objective of this article is to provide evidence-based recommendations for the optimal timing of surgical management of NSTI.
METHODS The MEDLINE database using PubMed was searched to identify English language articles published from January 1990 to September 2015 regarding adult and pediatric patients with NSTIs. A systematic review of the literature was performed, and the Grading of Recommendations Assessment, Development and Evaluation framework were used. A single population [P], intervention [I], comparator [C], and outcome [O] (PICO) question was applied: In patients with NSTI (P), should early (<12 hours) initial debridement (I) versus late (≥12 hours) initial debridement (C) be performed to decrease mortality (O)?
RESULTS Two hundred eighty-seven articles were identified. Of these, 42 papers underwent full text review and 6 were selected for guideline construction. A total of 341 patients underwent debridement for NSTI. Of these, 143 patients were managed with early versus 198 with late operative debridement. Across all studies, there was an overall mortality rate of 14% in the early group versus 25.8% in the late group.
CONCLUSION For NSTIs, we recommend early operative debridement within 12 hours of suspected diagnosis. Institutional and regional systems should be optimized to facilitate prompt surgical evaluation and debridement.
LEVEL OF EVIDENCE Systematic review/meta-analysis, level IV.
Necrotizing soft tissue infections (NSTI) are severe and rapidly progressing infections with extremely high morbidity and mortality rates. The term “necrotizing soft tissue infection” encompasses all types of infection involving any layers of the soft tissue including superficial fascia, deep fascia or muscle. The clinical manifestations range from pyoderma to necrotizing cellulitis, myositis, progressive bacterial synergistic gangrene, and life-threatening necrotizing fasciitis.[1]
Necrotizing soft tissue infections are commonly seen in the extremities and abdominal walls, although any part of the body can be affected. Fournier gangrene, for example, is a fulminant form of NSTI that involves the perineal, genital, or perianal regions in men. Even though there are several definitions and classification systems that have been used to describe these infections, they all have similar pathophysiologic, clinical characteristics, and share the same diagnostic and treatment strategies. Early diagnosis and treatment are essential, because the mortality ranges from 10% to 17% with some sources citing mortality rates up to 73%.[2][3]
Necrotizing soft tissue infections can be caused by polymicrobial (Type I) or monomicrobial organisms (Type II). Monomicrobial infections account for 10% of NSTI and are most commonly caused by Group A β-hemolytic streptococci, especially the toxin producing strains of S. pyogenes. Other less common organisms include Vibrio vulnificus (Type III NSTI), which is found in marine environments; Aeromonas hydrophila, found in fresh or brackish water; and Clostridium perfringens.[4] Polymicrobial infections account for the majority of infections and involve a combination of bacteria, including Staphylococcal, Streptococcal species, Escherichia coli, Bacteroides fragilis, or Clostridium species.[5]
There are no specific diagnostic criteria for NSTI as patients can present with a wide range of clinical findings. In fact, NSTI involving the fascial planes is often not recognized during the initial examination due to a paucity of skin findings, or markers of abscess or cellulitis.[6] Signs of systemic sepsis, including hypotension and tachycardia, may be present, although they are usually late findings. Laboratory values, such as serum C-reactive protein have low positive predictive values and radiologic findings such as gas within the soft tissue, are not reliably present. Although computed tomography and magnetic resonance imaging have a higher sensitivity than plain radiography, they lack specificity for the diagnosis of NSTI, and their use can lead to significant delays in definitive treatment. Despite the development of various scoring systems to facilitate the diagnosis of NSTI, the mainstay of diagnosis remains a high index of suspicion.[7–9]
Early, radical surgical debridement is fundamental in the treatment of NSTI and is associated with improved survival compared with delayed intervention. Multivariate analysis undertaken by Wong et al.[10] demonstrated that a delay in surgery of more than twenty-four hours correlated with increased mortality. Repeated debridement may be required, with several groups advising routine surgical reexploration within 24 hours.[11–13] Broad-spectrum antibiotics that include gram-negative, gram-positive, and anaerobic coverage should also be initiated immediately after the diagnosis is suspected and continued until adequate source control is achieved. Hyperbaric oxygen (HBO) therapy and intravenous immunoglobulin (IVIg) have been used as adjunctive strategies in the management of NSTI. Hyperbaric oxygen therapy delivers oxygen at two to three times atmospheric pressure, leading to high arterial and tissue oxygen tension. Although it is thought to have antibacterial effects, promote wound healing, and inhibit reperfusion injury, the use of HBO for NSTI is somewhat controversial.[14] Pooled IVIg binds certain bacterial exotoxins, limiting the systemic inflammatory response. Intravenous immunoglobulin has been shown to reduce mortality in sepsis and septic shock, but there are currently no data to support its routine use for NSTI.[15]
Although source control is a priority in the management of NSTI, early surgical intervention may be delayed due to prolonged intervals between symptom onset and presentation, particularly for patients requiring transfer to a specialized regional center. Making the correct diagnosis can be challenging, especially among immunocompromised patients that do not manifest typical clinical or laboratory signs of NSTI. Studies have shown that patients with malignancy or those undergoing treatment with steroids or chemotherapy are susceptible to delays in debridement and have a higher in-hospital mortality.[14][16] Patients that present in shock may be deemed too unstable to undergo immediate surgical intervention and admission to the intensive care unit (ICU) for aggressive resuscitation can lead to delays in surgical debridement.
Even though it has been established that early operative debridement is the mainstay of treatment for these aggressive infections, there is no consensus regarding the optimal timing of initial debridement. The purpose of this systematic review and meta-analysis was to create a practice management guideline for acute care surgeons regarding the optimal timing of initial debridement for NSTI, using a framework established by the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) Working Group.[17][18] The creation of this guideline was overseen by the Eastern Association for the Surgery of Trauma Practice Management Guideline Committee.
Objectives
The purpose of this practice management guideline was to define whether the timing of initial operation (early versus late) has an impact on patient outcomes. The population (P), intervention (I), comparator (C), and outcome (O) questions were defined as follows:
PICO QUESTION: In adult and pediatric patients with NSTI (P), should early (<12 hours) initial debridement (I) versus late (≥12 hours) initial debridement (C) be performed to decrease mortality (O)?
Materials and Methods
Identification of References
Inclusion criteria consisted of articles published in the English language that included adult and pediatric patients requiring surgical intervention for the management of NSTI. Letters to the editor, single-case reports, book chapters, and review articles were excluded.
Two professional librarians conducted a systematic search from January 1987 until September 2015 using the PubMed, EMBASE, and the Cochrane Library databases of published studies. The searches were done using Medical Subject Headings (MeSH) terms (Necrotizing[All Fields] AND (“fasciitis”[MeSH Terms] OR “fasciitis”[All Fields]) AND (“debridement”[MeSH Terms] OR “debridement”[All Fields]) AND (“methods” [Subheading] OR “methods”[All Fields] OR “methods”[MeSH Terms])) AND ((“necrosis”[MeSH Terms] OR “necrosis”[All Fields]) AND (“mortality”[Subheading] OR “mortality”[All Fields] OR “mortality”[MeSH Terms])) AND ((“time”[MeSH Terms] OR “time”[All Fields]) AND Factors[All Fields]) OR “necrosis” [MeSH Terms] OR “necrosis”[All Fields] AND (“debridement” [MeSH Terms] OR “debridement”[All Fields]) AND timing[All Fields]. In addition to the electronic search, we hand-searched the bibliographies of recent reviews and articles.
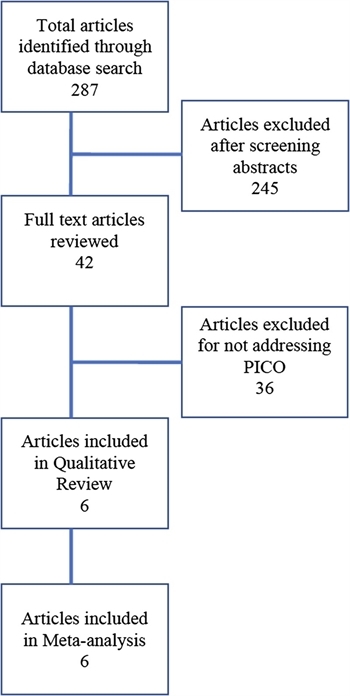
FIGURE 1. CONSORT diagram detailing literature search and article review
The electronic literature search identified 287 articles. Two independent reviewers then screened the titles and abstracts for the PICO inclusion criteria. Any disagreement on inclusion was resolved by consensus of the entire writing group. Two-hundred and forty-five studies were excluded as they were case reports. The remaining 42 studies underwent full-text review by two independent reviewers to determine appropriateness for inclusion. Thirty-five of these were excluded as they did not discuss surgical intervention for NSTI or did not address initial debridement. This yielded seven studies that addressed the PICO question for the creation of this guideline (Fig. 1).[12][19–24] However, one of these studies was excluded because the authors only reported median times from diagnosis to surgery and there was no comparison between early versus late debridement.[19] Therefore, a total of six studies were included in the analysis.
Outcome Measure Types
Potential outcomes were chosen by the committee as per the GRADE approach.[17][18] Outcomes that were considered included mortality, organ failure, sepsis, total number of debridements, hospital, and ICU lengths of stay. The only outcome deemed critical by unanimous vote of the committee members was mortality, and this was chosen as the primary outcome measure. Organ failure, sepsis, number of debridements, and hospital and ICU lengths of stay were considered important but noncritical. There was a paucity of data on these outcomes, and they were therefore not included.
Data Extraction and Management
Two committee members reviewed each of the final six articles and extracted the data pertaining to our PICO question into a spreadsheet for review (Excel, Microsoft Corporation, Redmond, WA).
Minor heterogeneity existed given differences in the patient populations and variability in the types of surgery performed for NSTI debridement. These differences across studies were examined to assess the clinical and methodological heterogeneity. Review Manager X.6 (RevMan5, Cochrane Community, London, United Kingdom) was used to calculate the Q statistic. The I2 statistic (%) was used to determine the proportion of variation between studies attributable to heterogeneity and categorized as low (25%–49%), moderate (50%–74%), or high (74%–100%). Final recommendations also considered the overall quality of the evidence, the balance between desirable and undesirable effects, patient values, and preferences, as well as resource utilization and cost estimates.
Results
Results for the Timing of NSTI Debridement Less Than 12 Hours After Suspected Diagnosis
PICO QUESTION: In adult and pediatric patients with NSTI (P), should early (<12 hours) initial debridement (I) versus late (≥12 hours) initial debridement (C) be performed to decrease mortality (O)?
Qualitative Synthesis
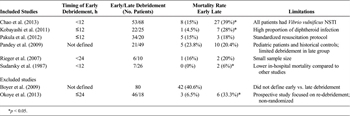
TABLE 1. Summary of Selected Studies
Our search yielded six studies that addressed the critical outcome of mortality following operative debridement for NSTI.[12][20–24] These are summarized in Table 1. There were no randomized controlled trials included in our analysis. A total of 143 patients were managed with early operative debridement (<12 hours) versus 198 in the late (≥12 hours) debridement group. Across all studies, there was an overall mortality rate of 14% in the early group versus 25.8% in the late group.
The largest of the retrospective studies by Chao et al.[20] compared the impact of early (<12 hours) debridement in 53 patients with V. vulnificus NSTI to late (≥12 hours after admission) debridement in 68 patients, and found a significant difference in mortality rates (15% vs. 39%, p = 0.037). One advantage of this study is that all patients had NSTI due to V. vulnificus thereby avoiding any confounding due to type of organism. It is important to note that in this study, all patients presented with sepsis and over 50% had septic shock on admission. Those who underwent late debridement were more likely to be hypotensive with higher APACHE II scores. It is possible that debridement was delayed in these patients due to their hemodynamic instability.
A retrospective study of 47 patients with NSTI by Kobayashi et al.[21] found that the mortality among patients undergoing early debridement (≤12 hours from the time of ED admission) was significantly lower than that of patients undergoing late (>12 hours) debridement (4.5% vs. 28%, p = 0.033). Delayed debridement was also associated with higher incidence of septic shock and acute kidney injury, as well as an increased number of debridements. This study was limited by the small number of patients. It is also worth noting that they had a high proportion of infections caused by diphtheroids which tend to cause more severe skin and soft tissue damage and therefore may be recognized earlier than NSTI caused by less aggressive organisms.
A retrospective analysis of 54 patients with NSTI by Pakula et al.[22] found that a delay to surgery (>12 hours from admission) did not impact mortality (15% for early vs. 18% for delayed, p = 1.0). Limitations of this study include its small sample size and heterogeneous patient population, as well as the fact that patients were not randomized to treatment groups. In fact, the majority of patients were managed with early debridement. However, all patients were resuscitated according to a standardized protocol, eliminating potential confounders due to variations in sepsis management.
Pandey et al.[23] conducted a retrospective study of 70 pediatric patients with NSTI. Patients underwent conservative debridement (limited to necrotic skin) 3 days to 5 days after starting antibiotics. These were compared to a group of historic controls (two years prior to the conservative group) that underwent early aggressive surgical debridement. The actual timing of the early debridement was never defined in this study, but it was reported to have occurred immediately after the diagnosis of NSTI was made. The mortality was not significantly different between the two groups (20.4% vs. 23.8% in the conservative and early groups, respectively; p > 0.05). The higher overall mortality reported in this study may have been because several of the patients were premature neonates. Many of the patients included in this study were also of lower socioeconomic status and suffered from malnutrition. Limitations of this study include the fact that exact timing of early aggressive debridement is not defined, it uses historical controls and the surgical therapy is described as “conservative debridement” which is generally not considered standard of care.
A small retrospective study of 16 patients with NSTI by Rieger et al.[24] found a mortality rate of 16% in the six patients undergoing early (<24 hours) debridement versus 20% in the 10 patients undergoing late (≥24 hours) debridement, although this did not reach statistical significance given the small sample size. In a retrospective study of 33 patients with NSTI by Sudarsky et al.,[12] patients who were debrided within 12 hours of admission had a lower mortality than those in whom debridement was delayed for 12 hours to 48 hours (0% vs. 6.1%, respectively). A third group of patients debrided after 48 hours also had a lower mortality rate, perhaps suggesting that they had a less aggressive form of the disease.
Quantitative Synthesis (Meta-analysis)
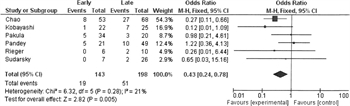
FIGURE 2. Forest plot for early (<12 h) vs. late (≥12 h) debridement in NSTI. Outcome = mortality
A comparison of early versus late debridement with mortality as an outcome was found in six of the studies. Analysis of the pooled data showed that early debridement was associated with lower mortality than late debridement (Fig. 2). There was a mild amount of heterogeneity (I [2] = 21%).
Grading the Evidence

TABLE 2. GRADE Profile for Early (<12 h) Versus Late (≥12 h) Debridement
The majority of data was retrospective in nature, and there were no randomized controlled trials included in this analysis. Applying the GRADE framework to the outcome of reduced mortality rates, the overall quality of evidence was very low due to retrospective nature of the studies being considered and the small number of studies fulfilling criteria. The evidence was downgraded for risk of bias and imprecision, and inconsistency in the definition of “early” debridement for NSTI (Table 2).
Recommendation
PICO QUESTION: In adult and pediatric patients with NSTI (P), should early (<12 hours) initial debridement (I) versus late (≥12 hours) initial debridement (C) be performed to decrease mortality (O)?

FIGURE 3. Summary of recommendations
We recommend that patients undergo early initial debridement for NSTI within 12 hours of diagnosis (very low quality of evidence, very large magnitude of effect) (Fig. 3).
Our recommendation considers the quality of evidence, balance between desirable and undesirable effects, patient values and preferences, as well as resource utilization and cost estimates. Despite the overall quality of evidence being very low, NSTI is a condition with a high morbidity and mortality. Patients often require prolonged hospital and ICU lengths of stay, making resource utilization and health care–related costs for NSTI quite high. We considered that most patients and hospitals would place a high value on avoidance of mortality and infectious complications related to delayed management of NSTI. These factors resulted in the formulation of a strong recommendation by the group. The desirable effects of adherence to our recommendation likely outweigh the undesirable effects.
Future Investigations
Despite significant advances in management as well as improved knowledge regarding NSTIs, establishing an early diagnosis remains challenging. Future research should be aimed at investigating the accuracy of imaging, developing predictive models and validating risk calculators to assist with the diagnosis of NSTI. Given that patients with NSTI often require multiple trips to the operating room for debridement and reconstruction, there is also a need to better define the optimal timing for redebridement.
There is currently no conclusive data on the use of adjunctive treatments such as HBO. Given the lack of proven benefit from HBO and its potential to limit or delay delivery of care, future studies should define which patients might benefit from this therapeutic modality. In addition, there is some data to suggest that when used in conjunction with surgical intervention and appropriate antimicrobial therapy, IVIg and biomodulators may provide a clinically significant treatment benefit. However, the data is inconsistent and more evidence is needed before such interventions are used on a routine basis.
Using these Guidelines in Clinical Practice
This guideline represents an overview of the literature regarding the timing of initial debridement for NSTI. The literature we reviewed supports early (<12 hours) initial debridement for NSTI in adult and pediatric patients to decrease mortality. There are currently no formal protocols for diagnosis and initial management of NSTI; however, there is evidence to suggest that protocolized management may lead to improved outcomes.[25] In-hospital protocols to guide rapid assessment of patients’ clinical status, early resuscitation, initiation of broad-spectrum antibiotics and to determine the need for transfer to a specialized regional center may help avoid delays in definitive care. Most patients with NSTI are initially seen at nonreferral centers that may not be equipped to provide the necessary level of care. This complex surgical problem should be treated at specialized regional centers with experts who are proficient in the diagnosis and management of NSTI.[25][26] If transfer to a larger tertiary care center is deemed necessary, transport systems should be in place to transfer the patient quickly and efficiently to avoid significant delays in surgical debridement.[26] The long-term management of NSTI requires a complex multidisciplinary approach that extends well beyond the operative and resuscitation period. Specialized centers should have readily available surgical and ICU teams, wound care specialists, nutritionists, and physical and occupational therapists, because coordination of care among all services is essential for achieving the best long-term outcomes in NSTI.
Authorship
P.F. served as EAST Guideline Section liaison. R.B.G., P.F., D.D.Y., M.L. formulated the PICO questions, conducted the literature search and screened titles and abstracts. R.B.G., P.F., D.D.Y., B.W. and M.L. performed the full text review. R.B.G., P.F., D.D.Y., B.W., M.L., J.Y., C.M., K.K., M.K., and A.K. abstracted data from selected articles. All authors appraised the evidence and contributed to the final recommendations. B.W. and P.F. contributed the GRADE table and forest plot. R.B.G. drafted the initial manuscript, which all authors critically revised.
Acknowledgment
We gratefully acknowledge Bryce H. Robinson, MD and Eileen Bulger, MD, from the University of Washington, Seattle, WA for their expertise and guidance.
Disclosure
The author declares no conflicts of interest.
Source of Funding: None
References
- Carroll PR, Cattolica EV, Turzan CW, McAninch JW. Necrotizing soft-tissue infections of the perineum and genitalia. Etiology and early reconstruction. West J Med. 1986;144:174–178.
- Faraklas I, Stoddard GJ, Neumayer LA, Cochran A. Development and validation of a necrotizing soft-tissue infection mortality risk calculator using nSQIP. J Am Chem Soc. 2013;217:153–161.
- Taviloglu K, Yanar H. Necrotizing fasciitis: strategies for diagnosis and management. World J Emerg Surg. 2007;2:19.
- Holtom PD. Necrotizing soft tissue infections. West J Med. 1995;163(6):568–569.
- Meade JW, Mueller CB. Necrotizing infections of subcutaneous tissue and fascia. Ann Surg. 1968;168(2):274–280.
- Patino J, Castro D. Necrotizing lesions of soft tissues: a review. World J Surg. 1991;15:235–239.
- Wall DB, Klein SR, Black S, de Virgilio C. A simple model to help distinguish necrotizing fasciitis from nonnecrotizing soft tissue infection. J Am Coll Surg. 2000;191(3):227–231.
- Wall DB, de Virgilio C, Black S, Klein SR. Objective criteria may assist in distinguishing necrotizing fasciitis from nonnecrotizing soft tissue infection. Am J Surg. 2000;179(1):17–21.
- Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (laboratory risk indicator for necrotizing fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535–1541.
- Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am. 2004;86(4):869.
- Kaiser R, Cerra FB. Progressive necrotizing surgical infections: a unified approach. J Trauma. 1981;21:349–355.
- Sudarsky LA, Laschinger JC, Coppa GF, Spencer FC. Improved results from a standardized approach in treating patients with necrotizing fasciitis. Ann Surg. 1987;206:661–665.
- Chelson J, Hasltensen A, Haga T, Hoiby EA. Necrotising fasciitis due to group A streptococci in western Norway: incidence and clinical features. Lancet. 1994;344:1111–1115.
- Hakkarainen TW, Kopari NM, Pham TN, Evans HL. Necrotizing soft tissue infections: review and current concepts in treatment, systems of care, and outcomes. Curr Probl Surg. 2014;51(8):344–362.
- Kaul R, McGeer A, Norrby-Teglund A, Kotb M, Schwartz B, O'Rourke K, Talbot J, Low DE. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome—a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis. 1999;28(4):800–807.
- Keung EZ, Liu X, Nuzhad A, Adams C, Ashley SW, Askari R. Immunocompromised status in patients with necrotizing soft-tissue infections. JAMA Surg. 2013;148(5):419–426.
- Callcut RA, Raja AS, Como J, Patel MB, Velopulos C, Chiu WC, Kerwin AJ, Lau BD, Ferrada P, Dahm P, et al. Writing an EAST Practice Management Guideline (PMG): A Step-By-Step How-To-Guide. 2015. https://www.east.org/education/treatment-guidelines/using-grade-in-east-practice-management-guidelines. Accessed March-22-2016.
- Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, Dahm P. Eastern Association for the Surgery of Trauma Practice Management Guidelines Ad Hoc Committee. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S283–S287.
- Boyer A, Vargas F, Coste F, Saubusse E, Castaing Y, Gbikpi-Benissan G, Hilbert G, Gruson D. Influence of surgical treatment timing on mortality from necrotizing soft tissue infections requiring intensive care management. Intensive Care Med. 2009;35:847–853.
- Chao WN, Tsai CF, Chang HR, Chan KS, Su CH, Lee YT, Ueng KC, Chen CC, Chen SC, Lee MC. Impact of timing of surgery on outcome of Vibrio vulnificus-related necrotizing fasciitis. Am J Surg. 2013;206(1):32–39.
- Kobayashi L, Konstantinidis A, Shackelford S, Chan LS, Talving P, Inaba K, Demetriades D. Necrotizing soft tissue infections: delayed surgical treatment is associated with increased number of surgical debridements and morbidity. J Trauma. 2011;71(5):1400–1405.
- Pakula AM, Kapadia R, Freeman B, Skinner RA. A 3-year experience with necrotizing fasciitis: favorable outcomes despite operative delays in a busy acute care hospital. Am Surg. 2012;78(10):1059–1062.
- Pandey A, Gangopadhyay AN, Sharma SP, Kumar V, Gopal SC, Gupta DK. Surgical considerations in pediatric necrotizing fasciitis. J Indian Assoc Pediatr Surg. 2009;14(1):19–23.
- Rieger UM, Gugger CY, Farhadi J, Heider I, Andresen R, Pierer G, Scheufler O. Prognostic factors in necrotizing fasciitis and myositis. Analysis of 16 consecutive cases at a single institution in Switzerland. Ann Plast Surg. 2007;58(5):523–530.
- Endorf FW, Klein MB, Mack CD, Jurkovich GJ, Rivara FP. Necrotizing soft-tissue infections: differences in patients treated at burn centers and non-burn centers. J Burn Care Res. 2008;29(6):933–938.
- Holena DN, Mills AM, Carr BG, et al. Transfer status: a risk factor for mortality in patients with necrotizing fasciitis. Surgery. 2011;150(3):363–370.
Keywords:
Necrotizing soft tissue infection; debridement; acute care surgery; Fournier gangrene; emergency general surgery; practice management guideline