Venous Thromboembolism Prophylaxis, Pediatric Trauma Patients - Joint between EAST and PTS
Published 2017
Citation: J Trauma. 82(3):627-636, March 2017
Authors
Mahajerin, Arash MD, MSCr; Petty, John K. MD; Hanson, Sheila J. MD, MS; Thompson, A. Jill PharmD; O’Brien, Sarah H. MD; Streck, Christian J. MD; Petrillo, Toni M. MD; Faustino, E. Vincent S. MD, MHS
Objectives
The primary objective of this guideline was to evaluate whether pharmacologic or mechanical prophylaxis reduces the incidence of VTE in children hospitalized after trauma and whether active surveillance with ultrasound (versus daily physical examination alone) results in earlier detection of VTE in this population. Our PICO (population [P], intervention [I], comparator [C], and outcome [O]) questions were as follows:
PICO Question 1
In children hospitalized after trauma (P), should pharmacologic VTE prophylaxis be utilized (I), compared with no pharmacologic prophylaxis (C), to reduce the incidence of VTE (O)?
PICO Question 2
In children hospitalized after trauma (P), should mechanical VTE prophylaxis be utilized (I), compared with no prophylaxis or in addition to pharmacologic prophylaxis (C), to reduce the incidence of VTE (O)?
PICO Question 3
In children hospitalized after trauma (P), should active surveillance for VTE with ultrasound be performed (I), compared with daily physical examination alone (C), to detect VTE earlier (O)?
A secondary objective was to evaluate putative risk factors for VTE in children hospitalized after trauma. The findings for this question were incorporated in PICO Question 1.
Inclusion Criteria for this Review
Study Types
We included case series, cross-sectional studies, case-control studies, cohort studies, and randomized controlled trials. Original studies from meta-analyses and reviews were also included. Case reports, surveys, and letters to the editor were excluded.
Participant Type
Any patient 0 to 21 years old who developed VTE after being hospitalized for trauma was included. Similar children who did not develop VTE were included as control subjects.
Intervention Types
Pharmacologic VTE prophylaxis consisted primarily of low-molecular-weight heparin, particularly enoxaparin, unfractionated heparin, or warfarin. Mechanical prophylaxis consisted of pneumatic compression devices or compression stockings. Ultrasound scans of the lower extremities and of insertion sites for central venous catheters were used for active surveillance for VTE. The putative risk factors evaluated were age, severity of injury, presence of central venous catheters, major surgery, site and type of injury (i.e., acute spinal cord, pelvis fracture, femur fracture, head injury, abdominal injury, and chest injury), obesity, mechanical ventilation, use of recombinant factor VIIa, and immobilization.
Outcome Measure Type
The relevant outcomes were the incidence of VTE for PICO Questions 1 and 2 and time to detection of VTE for PICO Question 3. Via consensus, the writing group considered incidence of VTE as a critical outcome and time to detection of VTE an important outcome. Venous thromboembolism was defined as deep vein thrombosis in the extremities and/or pulmonary embolism. For PICO Questions 1 and 2, only symptomatic VTE was included because this was the most consistently reported outcome in pediatric studies. For PICO Question 3, VTE detected by active surveillance with ultrasound, regardless of symptoms, was compared with symptomatic VTE. We also used symptomatic VTE as outcome for the review of putative risk factors. Given the paucity of data, other relevant outcomes (e.g., duration of hospitalization, incidence of stroke, mortality rate, duration of anticoagulation, recurrence of VTE, incidence of postthrombotic syndrome, and costs of care) were not evaluated, even though the writing group considered these as important outcomes.
Review Methods
Search Strategy
A medical librarian performed a systematic review of the MEDLINE database using from January 1946 to July 2015. The search strategies included “venous thromboembolism,” “trauma,” and “pediatric,” with additional subject headings and text words per concept and with added specific terms for “prophylaxis” and “prevention.” The search was restricted to humans, availability of full text article, and publication in English language. Only clinical studies in a pediatric trauma population, defined as 21 years or younger, or studies that combined adults and children but had delineated analyses for children were analyzed.
Study Selection/Data Extraction
Abstracts were reviewed for relevance to the PICO questions of interest by one of the authors (A.M.). Potentially relevant studies underwent full text review by the entire writing group to determine inclusion. Conflicts were resolved through group consensus. Once the included articles were determined, data on the study type, subject characteristics, presence of putative risk factors for VTE, type of prophylaxis, presence of VTE, and strength of association between exposure, that is, prophylaxis or putative risk factor, and VTE were extracted into a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, Washington). Data were checked in duplicate for accuracy by two members of the writing group assigned to the PICO question. Inconsistencies were resolved through full group review of the data and discussion.
Assessment of Methodological Quality/GRADE Process
The quality of evidence for each PICO question was assessed by two members of the writing group. Based on the GRADE guidelines, randomized controlled trials and observational studies were initially categorized as having high and low quality, respectively.[8] The category was upgraded or downgraded based on the five core GRADE domains of risk for bias, inconsistency, indirectness, imprecision, and publication bias, as well as the size of effect. The quality of evidence for each study was finalized after discussions with the entire writing group. We utilized GRADEpro (McMaster University and Evidence Prime Incorporated, Hamilton, Ontario, Canada), an online software, to create summary-of-findings tables for each PICO question.[10]
Measures of Treatment Effect
Because of the small number of studies available for each PICO question and significant differences in study design, meta-analysis was not performed, and summary measures of treatment effect were not calculated. Incidence of VTE was presented as counts (%), whereas that for time to detection of VTE was presented as median days. Comparisons for incidence of VTE were performed using Fisher exact test. For the putative risk factors, significant heterogeneity of studies or lack of control subjects prevented calculation of summary measures of effect. Strengths of association were expressed as odds or risk ratios.
Results
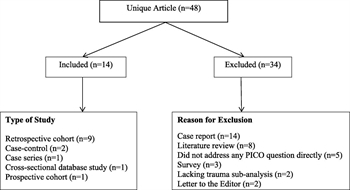
Figure 1. Flow diagram of included studies. Studies were excluded primarily based on the study design.
The literature search yielded 48 articles (Fig. 1). A total of 34 articles (71%) were excluded mainly because of study design. There were no randomized controlled trials. Of the included studies, only two addressed, at most partially, the PICO questions of interest.[11][12] A total of 14 studies addressed the putative risk factors for VTE.[3][6][7][12–22] The age ranges for these studies were 0 to 15 years old (one study), 0 to16 years old (one study), 0 to 17 years old (four studies), 0 to 18 years old (one study), 0 to 19 years old (one study), 0 to 20 years old (two studies), and 0 to 21 years old ( four studies).
Pharmacologic Prophylaxis in Children Hospitalized After Trauma (PICO Question 1)
In children hospitalized after trauma (P), should pharmacologic VTE prophylaxis be utilized (I), compared with no pharmacologic prophylaxis (C), to reduce the incidence of VTE (O)?
Qualitative Synthesis

TABLE 1. Summary of Findings for Prophylaxis Against VTE
The literature search yielded only one study with data on the incidence of VTE in children hospitalized after trauma who received pharmacologic prophylaxis compared with those who did not receive prophylaxis (Table 1). In this study, Hanson et al.[11] compared the incidence of VTE in children admitted to the intensive care unit after trauma during three time periods: prior to implementation of an institutional guideline for VTE prophylaxis using enoxaparin, rollout phase, and post–guideline implementation. The age ranges (median) of children in the three time periods were 0 to 18 (12) years old, 0 to 19 (10) years old, and 0 to 18 (7) years old, respectively. For purposes of this practice management guideline, only the preguideline and postguideline cohorts were included. In children at low risk of bleeding who received enoxaparin, 0 of 35 (0%) had symptomatic VTE compared with 9 of 308 children (2.9%) who did not receive enoxaparin (p = 0.75). This equated to 15 fewer (95% confidence interval [CI], 58 fewer to 28 more) VTE events per 1,000 critically ill children admitted after trauma. Low risk of bleeding in this study was defined as absence of intracranial bleeding, solid organ injury, planned surgical intervention or invasive procedure, and renal failure.
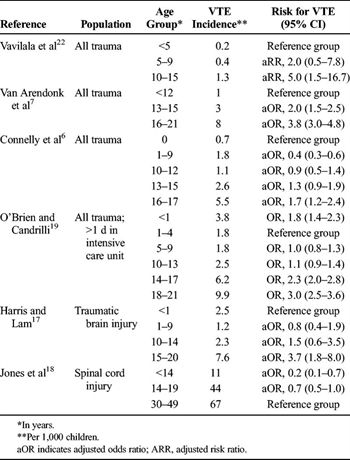
TABLE 2. Association of Age and VTE in Children Hospitalized After Trauma
In the absence of evidence to support a recommendation for
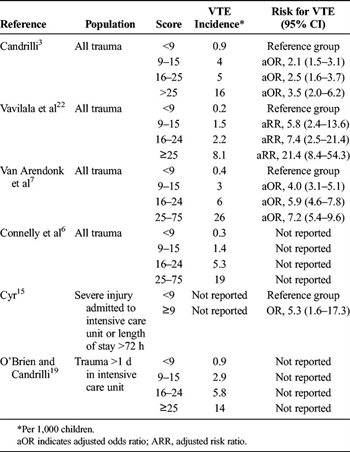
TABLE 3. Association of ISS and VTE in Children Hospitalized After Trauma
routine pharmacologic prophylaxis, we attempted to identify children hospitalized after trauma who are at high
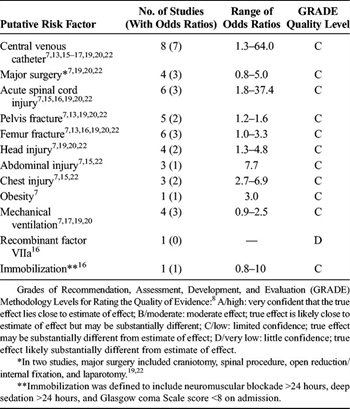
TABLE 4. Association of Other Putative Risk Factors and VTE in Children Hospitalized After Trauma
risk of VTE (Tables 2–4). Connelly et al.[6] published the first clinical prediction tool to predict VTE in children 0 to 17 years old who were hospitalized after trauma. The tool included Glasgow Coma Scale score, age, gender, intubation, admission to the intensive care unit, transfusion of blood products, placement of central venous catheter, pelvic and lower-extremity fracture, and major surgery. In derivation and validation cohorts, the tool performed well, with areas under the receiver operating characteristic curve greater than 0.90. A similar clinical prediction tool with similar findings was published while this guideline was under review.[23] The writing group was not able to critically appraise this publication or incorporate its findings into this guideline.
Adult trauma victims are known to be at risk of VTE, leading to concern that the risk of VTE in children hospitalized after trauma is higher after the onset of puberty.[24] Of four studies that analyzed children 0 years to 15 to 21 years old who were hospitalized after trauma, children older than 10 to 15 years (depending on the reference group) had increased risk of VTE (Table 2).[6][7][19][22] In a cohort of children 0 to 17 years old, Connelly et al.[6]demonstrated increased risk of VTE in children older than 15 years, but not for those 10 to 15 years old. In two additional database analyses of children with specific injuries, children 15 to 20 years old with traumatic brain injury had higher incidence of VTE compared with children 0 to 14 years old, whereas those 0 to 14 years old with acute spinal cord injury had lower incidence of VTE than did the reference adult group (30–49 years old).[17][18] In a recent national Delphi consensus study among pediatric trauma experts, consensus was reached that VTE prophylaxis should not be given to children 12 years or younger except in exceptional cases.[24]
Different thresholds of Injury Severity Score (ISS) have been used to define children at increased risk of VTE (Table 3). Among unselected children hospitalized after trauma, an ISS 25 or greater was strongly associated with VTE.[3][7][22] In subsets of children admitted to the intensive care unit or those with prolonged hospitalization, a score greater than 9 conferred higher risk of VTE.[15][19] The interaction between ISS and age of the child was not addressed in these studies. In the national Delphi consensus study, near-consensus was reached in favor of VTE prophylaxis in children with multiple major trauma or an ISS score greater than 25.[24]
The associations between the other putative risk factors, that is, central venous catheter, major surgery, site and type of injury (i.e., acute spinal cord, pelvis fracture, femur fracture, head injury, abdominal injury, and chest injury), obesity, mechanical ventilation, and immobilization, and VTE in children hospitalized after trauma, remain unclear (Table 4).[3][6][7][12][13][15–17][19][20][22] The odds ratios ranged from 0.8 to 64.0, depending on the putative risk factor. No studies provided the strength of association between the use of recombinant factor VIIa and VTE.
Grading the Evidence
With the use of the GRADE framework for evaluating the data specifically related to the outcome of incidence of VTE, very serious concerns about imprecision in the estimates were found (Table 1). Therefore, the overall quality of this evidence was downgraded from low to very low.
Discussion
An analysis of available evidence showed an absence of high-quality studies comparing the incidence of VTE in children hospitalized after trauma with respect to exposure to pharmacologic prophylaxis. The described work by Hanson et al.[11] showed a nonsignificant decrease in VTE with the use of enoxaparin in children at low risk of bleeding. The writing group excluded asymptomatic VTE identified by guideline-directed screening ultrasound in the analysis. It is possible that some of these VTE would eventually have become symptomatic. In adults with major trauma who are at low risk of bleeding, pharmacologic prophylaxis is recommended because of its proven efficacy and safety.[5] In addition, available evidence suggests that pharmacologic prophylaxis in children is relatively safe with regard to bleeding events, both after trauma and in other clinical settings.[25–27]
The available literature suggests that increasing age, particularly older than 15 years, and severe injury, defined as an ISS greater than 25, are associated with increased risk of VTE. In an attempt to combine the risks of VTE due to multiple factors, clinical prediction tools have been developed.[6][23] While these tools may improve our ability to identify the small cohort of children hospitalized after trauma who are at high risk of VTE, they do not provide any information on the efficacy and safety of pharmacologic prophylaxis.[28]Because of this significant gap in knowledge, the writing group unanimously decided to focus the recommendation only to children older than 15 years or those with an ISS greater than 25. Because of the maturation of the coagulation system during puberty, it is likely that the risk of VTE in younger postpubertal children approaches that of older children in the presence of an ISS greater than 25.[29][30] Similar extrapolations are not necessarily appropriate for prepubertal children with ISS greater than 25 but should be investigated. Onset of puberty, as defined by Tanner Stage 2 genital and pubic hair growth, has been shown to occur as early as 9 years in boys and 9.5 years in girls, depending on ethnicity.[31] The writing group agreed that it is possible that younger children or those with less severe injuries do not need pharmacologic prophylaxis, whereas children with multiple risk factors may need prophylaxis. However, with limited data on the efficacy and safety of pharmacologic prophylaxis in children, the writing group was unable to make any recommendations for these cohorts of children. Until such evidence becomes available, the writing group recommends that this guideline should be meant to provide a basic framework for the judicious use of pharmacologic prophylaxis in children hospitalized after trauma who are at highest risk of VTE. Similarly, based on current clinical practice, we suggest the use of enoxaparin over unfractionated heparin for pharmacologic prophylaxis.
Recommendation for Pharmacologic Prophylaxis in Children Hospitalized After Trauma (PICO Question 1)
We conditionally recommend that pharmacologic prophylaxis be considered for children older than 15 years who are at low risk of bleeding. We also conditionally recommend that pharmacologic prophylaxis be considered for children younger than 15 years old who are postpubertal if they have an ISS greater than 25. For prepubertal children, even with ISS greater than 25, we conditionally recommend against routine pharmacologic prophylaxis. Further studies are necessary to provide recommendations in prepubertal children. These recommendations are conditional, given the paucity of published data in children and the very low quality of the available evidence. Our recommendations are based on data in adults and the relative safety of enoxaparin at prophylactic doses in children.[5][25–27]
Mechanical VTE Prophylaxis in Children Hospitalized After Trauma (PICO Question 2)
In children hospitalized after trauma, should mechanical VTE prophylaxis be utilized, compared with no prophylaxis or in addition to pharmacologic prophylaxis, to reduce the incidence of VTE?
Qualitative Synthesis
The literature search yielded only two observational studies providing data on the incidence of VTE in children hospitalized after trauma who received mechanical prophylaxis compared with those who did not receive prophylaxis (Table 1). In the first study, Azu et al.[12] retrospectively compared the incidence of VTE in children admitted to a Level I trauma center for three age groups: Group 1 (0–12 years old), Group II (13–17 years old), and Group III (>17 years old). By local clinical practice, all children of adult size received mechanical prophylaxis, although its use was not documented. Children in Group I did not routinely receive any form of prophylaxis. Group II had pharmacologic prophylaxis ordered at the discretion of the attending surgeon. Its use was also not documented. Of the 1,025 children in Group II, two symptomatic VTE events occurred despite the presumed use of pneumatic compression device.
In the second observational study by Hanson et al.,[11] children at high risk of VTE and bleeding were recommended to receive pneumatic compression device per guideline. There were 60 children 0 to 18 years old in this category in the postguideline period, of whom 49 received the device only and 11 received pharmacologic prophylaxis after the bleeding risk diminished. A total of three VTE events, none of which were symptomatic, were identified in children who received the device only. The use of the device was not documented but was inferred from the guideline.
Neither study by Azu et al.[12] nor that of Hanson et al.[11] provided a comparable group of children who were not on any prophylaxis. To estimate the proportion of these children who developed VTE, we used data from the National Trauma Data Bank (NTDB) between 2000 and 2005 as reported by O’Brien and Candrilli.[19] Although NTDB does not record the use of pharmacologic or mechanical prophylaxis, the use of these interventions is uncommon in children.[13][32] The data from the 14- to 17-year age group was used to compare with the similarly aged Group II in the study by Azu et al.,[12] in which mechanical prophylaxis was used.
Based on these studies, 2 (0.2%) of 1,074 children who received mechanical prophylaxis had symptomatic VTE, whereas 215 (0.6%) of 34,451 children who did not receive mechanical prophylaxis (from the NTDB) had VTE (p = 0.08) (Table 1). This equated to 4 fewer (95% CI, 1–7 fewer) VTE events per 1,000 children hospitalized after trauma with mechanical prophylaxis versus no prophylaxis. In children who received pharmacologic prophylaxis in addition to mechanical prophylaxis, none of 11 children had symptomatic VTE, which was similar to none of 49 children solely on mechanical prophylaxis who had symptomatic VTE.
Grading the Evidence
With the use of the GRADE framework for evaluating the data specifically related to the outcome of incidence of VTE, very serious concerns about risk for bias, inconsistency, indirectness, imprecision in the estimates, and lack of control for potential confounders were found (Table 1). Therefore, the overall quality of this evidence was downgraded from low to very low.
Discussion
Analysis of available evidence showed an absence of high-quality studies comparing the incidence of VTE in children hospitalized after trauma with respect to exposure to mechanical prophylaxis. Inferred from the studies by Azu et al.[12] and Hanson et al.,[11] the use of mechanical, versus no prophylaxis, suggested a possible reduction on the incidence of VTE.[11][12] This effect is strengthened by data in adults showing significant reduction in the incidence of VTE after trauma with mechanical prophylaxis.[33] The writing group also gave weight to tolerability and safety of mechanical VTE prophylaxis. Mechanical prophylaxis was well accepted by pediatric providers in a multinational study with 24% of children 8 to 18 years old who were admitted to the intensive care unit receiving mechanical prophylaxis.[32]
Based on limited pediatric evidence, children on mechanical prophylaxis had similar risk of VTE compared with those on pharmacologic prophylaxis in addition to mechanical prophylaxis.[11] In adults who were hospitalized after trauma, pharmacologic prophylaxis was superior to mechanical prophylaxis in reducing the incidence of VTE.[5] Based on the potential benefits and relative safety of mechanical prophylaxis in children, it is reasonable to use mechanical prophylaxis alone or in addition to pharmacologic prophylaxis in children hospitalized after trauma at high risk of VTE. A potential limitation is the availability of appropriately sized sleeves for younger children.
Recommendation for Mechanical Prophylaxis in Children Hospitalized After Trauma (PICO Question 2)
We conditionally recommend that mechanical prophylaxis be considered alone or in addition to pharmacologic prophylaxis to hospitalized children older than 15 years and children younger than 15 years who are postpubertal if they have an ISS greater than 25 for whom an appropriately sized device is available. This recommendation is conditional, given the paucity of published data in children and the very low quality of the available evidence. Our recommendation is based on data in adults and the safety and tolerability of mechanical prophylaxis in children.[5][32]
Active Ultrasound Surveillance for VTE in Children Hospitalized After Trauma (PICO Question 3)
In children hospitalized after trauma, should active surveillance for VTE with ultrasound be performed, compared with daily physical examination alone, to detect VTE earlier?
Qualitative Synthesis
A single prospective study by Hanson et al.[11] incorporated screening ultrasound as part of a larger clinical care guideline in children 0 to 18 years old at high risk of VTE after admission to the intensive care unit after trauma (Table 1). Active surveillance with ultrasound was utilized in children at high risk of VTE and also at high risk of bleeding that would prevent safe use of pharmacologic prophylaxis. Children received ultrasound of both lower extremities and the upper extremity in which a central venous catheter was inserted on hospital Day 7 if they were still in the intensive care unit. Of 60 eligible children, 26% received ultrasound. Compared with unselected historical control subjects who did not have active surveillance with ultrasound, the guideline care group had asymptomatic VTE detected 3 days earlier. The published results did not allow for comparison of the high-risk group of children who received active surveillance to a comparable high-risk control group.
Grading the Evidence
With the use of the GRADE framework for evaluating the data specifically related to the outcome of time to detection of VTE, very serious concerns about risk for bias and lack of control for potential confounders were found (Table 1). Therefore, the overall quality of this evidence was downgraded from low to very low.
Discussion
The value of active surveillance for VTE with ultrasound in children hospitalized after trauma is unclear. The single prospective study that incorporated active surveillance with ultrasound as part of a larger care guideline showed an earlier diagnosis of VTE, although all of these events were asymptomatic.[11] While it seems intuitive that active surveillance with ultrasound would detect VTE before it becomes symptomatic, the timing, frequency, and extent required of such a strategy is unclear. Furthermore, the natural history of asymptomatic VTE in children is poorly described. Earlier diagnosis of VTE with active ultrasound surveillance may lead to increased use of therapeutic anticoagulation without clear benefit. In adults, active surveillance for DVT with ultrasounds was not efficacious in reducing the risk of symptomatic VTE.[34] It may, in fact, increase the risk of bleeding with therapeutic anticoagulation for any detected asymptomatic DVT. The risk of major bleeding with therapeutic anticoagulation in children can be as high as 24% with unfractionated heparin and 4% with enoxaparin.[2][35] In addition, the cost of widespread active surveillance with ultrasound for these uncommon events would need to be considered before general utilization could be recommended.
Recommendation for Active Ultrasound Surveillance for VTE in Children Hospitalized After Trauma (PICO Question 3)
We conditionally recommend against active surveillance for VTE with ultrasound for earlier detection of VTE compared with routine daily physical examination alone in children hospitalized after trauma. The potential benefits of earlier detection and treatment of VTE are unclear, but the risk of bleeding with therapeutic anticoagulation is well documented.
Further Investigation
The detailed review of the literature performed for this practice management guideline highlighted the paucity of data on VTE in children hospitalized after trauma. The evidence for pharmacologic and mechanical VTE prophylaxis in children is very low compared with adults.[36] Despite the developmental differences in the hemostatic system between children and adults that may affect the incidence of VTE and response to therapy, the writing group had to consider adult evidence in making the recommendations.[2]There is minimal evidence regarding effective risk stratification in children hospitalized after trauma. An initial approach for identifying children suitable for prophylaxis would be to focus on children older than 15 years. Clinical prediction tools should be easy to calculate at the bedside and should avoid risk markers such as ISS that are impracticable and lack acute pragmatic value. Risk stratification studies and subsequent randomized controlled trials are urgently needed to define the efficacy and safety of prophylaxis against VTE in children hospitalized after trauma. Because the numbers of critically injured patients and VTE are lower in children than in adults, traditional study designs based on the frequentist approach may not be feasible. The Bayesian approach, in which data from adults are formally incorporated in the design and adaptive randomization is used, may result in a smaller sample size and increase the likelihood of successfully completing the trial.[28]
The incidence of VTE was the only outcome evaluated in this guideline. The effect of VTE prophylaxis on other outcomes, such as duration of hospitalization, incidence of stroke, mortality rate, duration of anticoagulation, recurrence of VTE, incidence of postthrombotic syndrome, and costs of care, should be studied. The risks, sites, and severity of bleeding with VTE prophylaxis should also be explored.
Using These Guidelines in Clinical Practice
These guidelines represent a detailed summary of the limited literature regarding VTE prophylaxis in children hospitalized after trauma. The available evidence is of very low quality and observational in nature. As such, evidence from adults was considered in the writing group’s recommendations. These guidelines are intended to inform the decision-making process rather than replace clinical judgment.
Conclusions
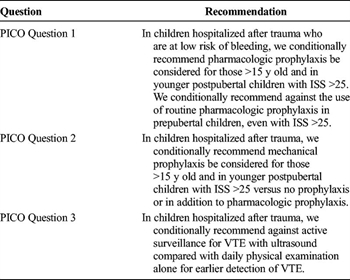
TABLE 5. Summary of Recommendations
In summary, we have provided evidence-based recommendations using the GRADE methodology (Table 5). First, in children hospitalized after trauma who are at low risk of bleeding, we conditionally recommend the use of pharmacologic prophylaxis be considered in children older than 15 years and in younger postpubertal children with ISS greater than 25. We conditionally recommend against the use of routine pharmacologic prophylaxis in prepubertal children, even with ISS greater than 25. Second, in children hospitalized after trauma, we conditionally recommend mechanical prophylaxis versus no prophylaxis or in addition to pharmacologic prophylaxis be considered in children older than 15 years and in younger postpubertal children with ISS greater than 25. Lastly, in children hospitalized after trauma, we conditionally recommend against active surveillance for VTE with ultrasound compared with routine daily physical examination alone for earlier detection of VTE.
Authorship
A.M. conducted initial review of potential articles. Data collection from selected articles was divided among the entire author group. Data analysis and interpretation were done by the entire author group. A.M. and E.V.S.F. wrote the initial draft of the manuscript, and all authors contributed to revisions.
Acknowledgement
The authors would like to acknowledge the Pediatric Trauma Society (PTS) for supporting the work of the authors, who comprise the PTS VTE workgroup. The PTS provided administrative and technical support for this project. The authors would also like to acknowledge the assistance provided by Drs. Bryce Robinson, Nicole Fox, and Yngve Falck-Ytter in developing this practice management guideline. Lastly, the authors would like to acknowledge Danielle Linden, medical librarian of the Burlew Library at St. Joseph Hospital of Orange, California, for her expert assistance in the literature search.
Disclosure
S.J.H. is a site investigator for an anticoagulation trial sponsored by Bristol-Myers Squibb. S.H.O. is the principal investigator and a steering committee member for anticoagulation trials sponsored by Bristol-Myers Squibb and Pfizer. S.H.O. is also a member of the data and safety monitoring board for GlaxoSmithKline. E.V.S.F. is a member of the data and safety monitoring board for an anticoagulation trial sponsored by GlaxoSmithKline and is partially supported by a grant from the American Heart Association.
References
- Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001–1008.
- Monagle P, Chan AK, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Gottl U, Vesely SK. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(Suppl 2):e737S–e801S.
- Candrilli SD, Balkrishnan R, O’Brien SH. Effect of injury severity on the incidence and utilization-related outcomes of venous thromboembolism in pediatric trauma inpatients. Pediatr Crit Care Med. 2009;10(5):554–557.
- Goudie A, Dynan L, Brady PW, Fieldston E, Brilli RJ, Walsh KE. Costs of venous thromboembolism, catheter-associated urinary tract infection, and pressure ulcer. Pediatrics. 2015;136(3):432–439.
- Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(Suppl 2):e227S–e277S.
- Connelly CR, Laird A, Barton JS, Fischer PE, Krishnaswami S, Schreiber MA, Zonies DH, Watters JM. A clinical tool for the prediction of venous thromboembolism in pediatric trauma patients. JAMA Surg. 2016;151(1):50–57.
- Van Arendonk K, Schneider E, Haider A, Colombani P, Stewart F, Haut E. Venous thromboembolism after trauma: when do children become adults? JAMA Surg. 2013;148(12):1123–1130.
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–382.
- Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, Dahm P. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S283–S287.
- GRADEpro [computer program] [http://www.gradepro.org/]. Version January 15, 2014. Hamilton, Ontario, Canada: McMaster University; 2014.
- Hanson SJ, Punzalan RC, Arca MJ, Simpson P, Christensen MA, Hanson SK, Yan K, Braun K, Havens PL. Effectiveness of clinical guidelines for deep vein thrombosis prophylaxis in reducing the incidence of venous thromboembolism in critically ill children after trauma. J Trauma Acute Care Surg. 2012;72(5):1292–1297.
- Azu MC, McCormack JE, Scriven RJ, Brebbia JS, Shapiro MJ, Lee TK. Venous thromboembolic events in pediatric trauma patients: is prophylaxis necessary? J Trauma. 2005;59(6):1345–1349.
- Askegard-Giesmann J, O’Brien S, Wang W, Kenney B. Increased use of enoxaparin in pediatric trauma patients. J Pediatr Surg. 2012;47(5):980–983.
- Cook A, Shackford S, Osler T, Rogers F, Sartorelli K, Littenberg B. Use of vena cava filters in pediatric trauma patients: data from the National Trauma Data Bank. J Trauma. 2005;59:1114–1120.
- Cyr C, Michon B, Pettersen G, David M, Brossard J. Venous thromboembolism after severe injury in children. Acta Haematol. 2006;115(3–4):198–200.
- Hanson SJ, Punzalan RC, Greenup RA, Liu H, Sato TT, Havens PL. Incidence and risk factors for venous thromboembolism in critically ill children after trauma. J Trauma. 2010;68(1):52–56.
- Harris DA, Lam S. Venous thromboembolism in the setting of pediatric traumatic brain injury. J Neurosurg Pediatr. 2014;13(4):448–455.
- Jones T, Ugalde V, Franks P, Zhou H, White RH. Venous thromboembolism after spinal cord injury: incidence, time course, and associated risk factors in 16,240 adults and children. Arch Phys Med Rehabil. 2005;86(12):2240–2247.
- O’Brien SH, Candrilli SD. In the absence of a central venous catheter, risk of venous thromboembolism is low in critically injured children, adolescents, and young adults: evidence from the National Trauma Data Bank. Pediatr Crit Care Med. 2011;12(3):251–256.
- O’Brien SH, Klima J, Gaines BA, Betz S, Zenati MS. Utilization of low-molecular-weight heparin prophylaxis in pediatric and adolescent trauma patients. J Trauma Nurs. 2012;19(2):117–121.
- Truitt AK, Sorrells DL, Halvorson E, Starring J, Kurkchubasche AG, Tracy TF Jr, Luks FI. Pulmonary embolism: which pediatric trauma patients are at risk? J Pediatr Surg. 2005;40(1):124–127.
- Vavilala MS, Nathens AB, Jurkovich GJ, Mackenzie E, Rivara FP. Risk factors for venous thromboembolism in pediatric trauma. J Trauma. 2002;52(5):922–927.
- Yen J, van Arendonk KJ, Streiff MB, McNamara L, Stewart FD, Conner KG, Thompson RE, Haut ER, Takemoto CM. Risk factors for venous thromboembolism in pediatric trauma patients and validation of a novel scoring system: the risk of clots in kids with trauma score. Pediatr Crit Care Med. 2016;17(5):391–399.
- Hanson SJ, Faustino EV, Mahajerin A, O’Brien SH, Streck CJ, Thompson AJ, Petrillo TM, Petty JK. Recommendations for venous thromboembolism prophylaxis in pediatric trauma patients: a national, multidisciplinary consensus study. J Trauma Acute Care Surg. 2016;80(5):695–701.
- Bidlingmaier C, Kenet G, Kurnik K, Mathew P, Manner D, Mitchell L, Krumpel A, Nowak-Gottl U. Safety and efficacy of low molecular weight heparins in children: a systematic review of the literature and meta-analysis of single-arm studies. Semin Thromb Hemost. 2011;37(7):814–825.
- Stem J, Christensen A, Davis D, Raffini L. Safety of prophylactic anticoagulation at a pediatric hospital. J Pediatr Hematol Oncol. 2013;35(7):e287–e291.
- Thompson AJ, McSwain SD, Webb SA, Stroud MA, Streck CJ. Venous thromboembolism prophylaxis in the pediatric trauma population. J Pediatr Surg. 2013;48(6):1413–1421.
- Faustino EV. It’s time to ROCKIT: predicting venous thrombosis in children after trauma. Pediatr Crit Care Med. 2016;17(5):458–459.
- Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L. Maturation of the hemostatic system during childhood. Blood. 1992;80:1998–2005.
- Monagle P, Barnes C, Ignjatovic V, Furmedge J, Newall F, Chan A, de Rosa L, Hamilton S, Ragg P, Robinson S, et al. Developmental haemostasis; impact for clinical laboratories. Thromb Haemost. 2006;95:362–372.
- Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sørensen TI, Dunkel L, Himes JH, Teilmann G, Swan SH. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121:S172–S191.
- Faustino EV, Hanson S, Spinella PC, Tucci M, O’Brien SH, Nunez AR, Yung M, Truemper E, Qin L, Li S, et al. A multinational study of thromboprophylaxis practice in critically ill children. Crit Care Med. 2014;42(5):1232–1240.
- Barrera LM, Perel P, Ker K, Cirocchi R, Farinella E, Morales Uribe CH. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013;3:CD008303.
- Robinson KS, Anderson DR, Gross M, Petrie D, Leighton R, Stanish W, Alexander D, Mitchell M, Flemming B, Gent M. Ultrasonographic screening before hospital discharge for deep venous thrombosis after arthroplasty: the post-arthroplasty screening study. A randomized, controlled trial. Ann Intern Med. 1997;127:439–445.
- Dix D, Andrew M, Marzinotto V, Charpentier K, Bridge S, Monagle P, deVeber G, Leaker M, Chan A, Massicotte P. The use of low molecular weight heparin in pediatric patients: a prospective cohort study. J Pediatr. 2000;136:439–445.
- Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, Cook DJ, Balekian AA, Klein RC, Le H, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(Suppl 2):e195S–e226S.