Elderly Adults with Isolated Hip Fractures - Orthogeriatric Care versus Standard Care
Published 2020
Citation: J Trauma. 88(2):266-278, February 2020
Visual PMG
Authors
Mukherjee, Kaushik MD, MSCI; Brooks, Steven E. MD; Barraco, Robert D. MD; Como, John J. MD, MPH; Hwang, Franchesca MD; Robinson, Bryce R. H. MD, MS; Crandall, Marie L. MD, MPH
Author Information
From the Division of Acute Care Surgery (K.M.), Loma Linda University Medical Center, Loma Linda, California; Division of Trauma and Surgical Critical Care (S.E.B.), Texas Tech University Health Sciences Center, Lubbock, Texas; Trauma-Surgical Critical Care/General Surgery (R.D.B.), Lehigh Valley Health Network, Allentown, Pennsylvania; Division of Trauma (J.J.C.), MetroHealth Medical Center, Cleveland, Ohio; Department of Surgery (F.H.), Rutgers Medical School, Newark, New Jersey; Department of Surgery (B.R.H.R.), Harborview Medical Center, University of Washington School of Medicine, Seattle, Washington; and Department of Surgery (M.L.C.), University of Florida College of Medicine, Jacksonville, Florida.
Submitted: July 3, 2019, Accepted: July 19, 2019, Published online: August 28, 2019.
Presented at Eastern Association for the Surgery of Trauma 32[nd] Annual Scientific Assembly, January 15–19, 2019, Austin, Texas.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
Address for reprints: Marie E. Crandall, MD, MPH, FACS, Department of Surgery, University of Florida College of Medicine Jacksonville, 655 W. 8[th] Street, Jacksonville, FL 32209; email: marie.crandall@jax.ufl.edu.
Online date: August 29, 2019
Abstract
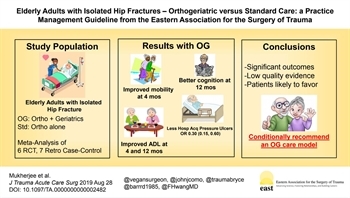
BACKGROUND: Elderly patients commonly suffer isolated hip fractures, causing significant morbidity and mortality. The use of orthogeriatrics (OG) management services, in which geriatric specialists primarily manage or co-manage patients after admission, may improve outcomes. We sought to provide recommendations regarding the role of OG services.
METHODS: Using GRADE methodology with meta-analyses, the Practice Management Guidelines Committee of the Eastern Association for the Surgery of Trauma conducted a systematic review of the literature from January 1, 1900, to August 31, 2017. A single Population, Intervention, Comparator and Outcome (PICO) question was generated with multiple outcomes: Should geriatric trauma patients 65 years or older with isolated hip fracture receive routine OG management, compared with no-routine OG management, to decrease mortality, improve discharge disposition, improve functional outcomes, decrease in-hospital medical complications, and decrease hospital length of stay?
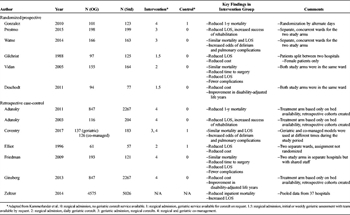
RESULTS: Forty-five articles were evaluated. Six randomized controlled trials and seven retrospective case-control studies met the criteria for quantitative analysis. For critical outcomes, retrospective case-control studies demonstrated a 30-day mortality benefit with OG (OR, 0.78 [0.67, 0.90]), but this was not demonstrated prospectively or at 1 year. Functional outcomes were superior with OG, specifically improved score on the Short Physical Performance Battery at 4 months (mean difference [MD], 0.78 [0.28, 1.29]), and improved score on the Mini Mental Status Examination with OG at 12 months (MD, 1.57 [0.40, 2.73]). Execution of activities of daily living was improved with OG as measured by two separate tests at 4 and 12 months. There was no difference in discharge disposition. Among important outcomes, the OG group had fewer hospital-acquired pressure ulcers (OR, 0.30 [0.15, 0.60]). There was no difference in other complications or length of stay. Overall quality of evidence was low.
CONCLUSION: In geriatric patients with isolated hip fracture, we conditionally recommend an OG care model to improve patient outcomes.
LEVEL OF EVIDENCE: Systematic review/meta-analysis, level III.
Overview
In the year 2000, the geriatric subgroup represented 12% of the American population. By the year 2050, this proportion will increase to over 20%.[1] Geriatric patients comprise more than 20% of hospital admissions and admissions at major trauma centers.[2][3] This aging population will have a profound impact on both outcome and cost of trauma care.[3] Geriatric trauma already accounts for 33% of trauma care expenditures in the United States, or US $9 billion per year,[4] while trauma ranks as the seventh-highest cause of death for those 65 years and older.[5]
Among elderly trauma patients, ground-level fall (GLF) is the most common traumatic mechanism, occurring nearly 10 times more often than motor vehicle crashes.[6] Nearly one in three geriatric persons will have a GLF each year.[7] These GLFs are not benign in this population, as 6% will sustain a fragility fracture, defined as a fracture resulting from standing height or less.[8] As many as 10% to 30% of GLF patients will incur multiple trauma, and mortality may reach 7%.[7]
Isolated hip fractures, most commonly caused by GLFs, prompt 340,000 hospitalizations annually in the United States with an associated cost of nearly US $3 billion per year. Additionally, hip fractures are expected to increase to 500,000 per annum in the United States by 2050.[9] In a study of over 25,000 geriatric trauma patients in 127 hospitals, Maxwell and colleagues found that 56% had a major operative procedure. Thirty-six percent of these patients had femoral neck fractures, the most common injury.[10] Mortality associated with hip fractures is 5% to 10% in the first 30 postoperative days and 12% to 37% within the first year after surgery. Hip fracture patients have five- to eight-fold increased mortality risk in the 3 months following their trauma, have functional and self-care limitations, and suffer decreased strength and altered balance, increasing the risk for additional falls.[11] Furthermore, one third of hip fracture patients have reduced cognitive function.[12] Concussion or traumatic brain injury may complicate recovery for the geriatric fragility fall patient by reducing functional independence, decreasing activities of daily living (ADLs), and by creating deficits in cognition, behavior, and motor skills.[11]
Statement of the Problem
Ideal treatment of the geriatric trauma patient with a hip fragility fracture, or hip fracture after GLF, includes reducing all modifiable risk factors, optimizing the patient for general anesthesia and surgery, efficiently completing definitive surgical care, rounding daily with a multidisciplinary team, managing medical comorbidities, reducing polypharmacy, planning early for discharge, and transitioning smoothly to posthospital care. This model necessitates the participation of trauma surgeons, medical physicians or geriatricians, orthopedic surgeons, pharmacists, respiratory therapists, nurses, physical therapists, occupational therapists, social workers, case managers, palliative care specialists, and advanced practice providers. Overall, coordinated multidisciplinary care has improved outcomes in these fragile, fracture patients.[13][14] With many teams involved in the care of these older trauma patients, questions arise as to which team should provide leadership and coordination of care, how the multidisciplinary care approach can be organized and managed, and the best physical location in the hospital for these patients.
Evidence-based answers to these questions have not yet appeared in the literature. In fact, among academic trauma surgeons, there exists disparate opinions as to whether fragility fractures in general, or hip fractures specifically, are even worthy of admission to a dedicated trauma service.[15] Numerous models for shared care of these patients have been described. Kammerlander and colleagues identified four models of multidisciplinary care for elderly patients with hip fracture based on literature review, which are adapted below and include[12]:
- 0. Admission to surgical ward with no geriatric consulting service available.
- 1. Admission to orthopedic ward with geriatric consulting service upon request.
- 1.5. Admission to surgical ward with initial or weekly geriatric assessment with team available by request.
- 2. Admission to orthopedic ward with daily geriatric consulting service and geriatric participation from admission to discharge (most common model).
- 3. Admission to geriatric/rehabilitation ward with orthopedic consultative service (on request).
- 4. Admission to orthopedic ward utilizing integrative care; orthopedic surgery and geriatrics co-manage the patient from admission until discharge
Our goal was to provide recommendations for the use of orthogeriatric (OG) services, defined as involvement of a medical physician or geriatrician in daily trauma care, by comparing outcomes for OG care versus traditional care. Traditional care is defined in the above schema as either 0 or 1. The OG services were defined as 1.5 to 4 on the above schema, with the distinction being the increased availability of the geriatric consulting service in 1.5 versus 1.
Methods
PICO Question Generation
In following GRADE methodology,[16] our team generated PICO questions. Multiple potential outcomes of interest were posited, including resource allocation, clinical outcomes, and hospital charges. Each person voted on each outcome using a nine-point Likert scale to determine critical outcomes, which all had a mean score of 7 or higher. Outcomes not felt to be critical by the authors were all felt to be important and were thus classified. We limited the review to studies in which our critical outcomes (mortality, discharge disposition, and independence/long-term functional outcomes) or our important outcomes (hospital length of stay [LOS] and in-hospital medical complications) were studied. Our PICO question was:
Population: Geriatric trauma patients 65 years or older with isolated hip fracture.
Intervention: OG management (adapted Kammerlander classification 0–1) 1.5–4.
Comparator: Traditional care (adapted Kammerlander classification 1.5–4) 0–1
Outcomes: Mortality (critical), discharge disposition (critical), functional outcomes (critical), in-hospital medical complications (important), and hospital LOS (important)
Inclusion Criteria for This Review
Study Types
Studies included prospective randomized controlled trials (RCT) and retrospective case-control studies (RCCS). Case reports, case series, retrospective before/after studies, research protocols, studies without comparative data, and reviews containing no original data or analyses were excluded. We also excluded editorials, opinion articles, and studies not addressing the PICO question. We included all studies published between January 1, 1900, and August 31, 2017. We did not restrict by publication language but excluded articles without an English translation.
Participant Types
We included all relevant studies, irrespective of race, sex, or other demographic characteristics.
Intervention Types
We reviewed all studies which compared outcomes for an OG model of care versus traditional models of care. For purposes of this review, an OG model was defined as one that had a geriatrician routinely caring for the daily needs of geriatric trauma patients. Traditional models of care included either no availability for geriatric consultation or having a geriatric consult available only upon request with no regular continued availability once consulted.
Review Methods
Search Strategy
In September 2017, an institutional research librarian performed a systematic search of Ovid, MEDLINE, Embase, and Web of Science. Supplemental Digital Content 1, Appendix 1, http://links.lww.com/TA/B475 contains the MeSH terms used for the initial search.
Study Selection
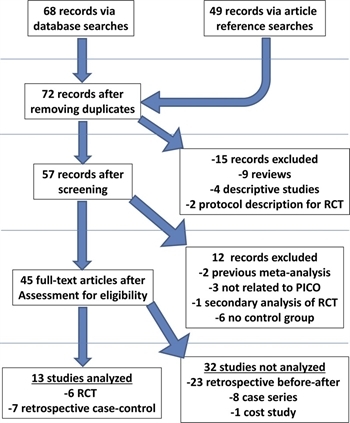
Figure 1: A standard PRISMA flow diagram is depicted above, illustrating the flow of the literature search and analysis algorithm.
Two independent reviewers (M.E., M.C.) screened the references by title and abstract and all non-relevant articles, editorials, case reports, and duplicates were removed. We then screened references for each article and added pertinent articles to the total. The resulting studies were used for this review. This process is highlighted in the PRISMA flow diagram (Fig. 1).
Data Extraction and Management
All references used for the review were loaded onto a Google Drive (Google LLC, Menlo Park, CA). All articles, GRADE resources, and instructions were electronically available to all members of the writing team. Each independent reviewer shared his or her PICO sheet and literature review with all members of the team. Independent interpretations of the data were shared through group email and conference calls. No reviewer discrepancies occurred; had any discrepancies occurred, the corresponding author would have adjudicated the conflict after discussion among all parties via teleconference. Data extraction was completed in July 2018.
Methodological Quality Assessment
We used GRADE methodology for this study.[16] Each designated reviewer independently evaluated the aggregate data with respect to the quality of the evidence to adequately answer each PICO question and quantified the strength of any recommendations. Reviewers were asked to determine effect size, risk of bias, inconsistency, indirectness, precision, and publication bias.
Recommendations were based on the overall quality of the evidence. Language for recommendations used the wording “we recommend” for strong recommendations, and “we conditionally recommend” for weaker recommendations.
Statistical Analysis
Specific comparisons were made to formulate data on the following outcomes: inpatient/30-day mortality, 1-year mortality, hospital LOS, likelihood of discharge to home, in-hospital acquired medical complications (including a specific analysis of hospital-acquired pressure ulcers), and functional outcomes at 4 months and 12 months, including cognitive performance (Mini-Mental Status Examination), mobility (Short Physical Performance Battery), and execution of ADLs (Barthel and Nottingham Extended ADL scales). The decision to use functional assessments was made a priori with the specific functional assessments performed dependent on the literature available. For dichotomous outcomes, data were pooled by entering data into Review Manager 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The Mantel-Haenszel statistical method was used with a random effects model and the odds ratio (OR) was used as the effect measure. For continuous variables, Review Manager was also used with an inverse variance method and a random effects model to yield mean difference as an effect measure.[17][18] When data were reported in articles as a mean and standard deviation (SD) for continuous variables, such data were entered directly. When data were reported as mean with 95% confidence interval (95% CI), we assumed that SD = 95% CI/3.92. When data were reported as median and interquartile range (IQR), the assumption was made that the mean and median were equivalent and the approximation SD = IQR/1.35 was used to yield an estimated SD. In one study,[19] two different OG care models were used; the data from these two models were pooled to compare with the standard arm. This study also reported data as median and IQR; the mean of the IQR for the two studies was used as an approximation for the IQR of the pooled group, which was then inserted into the approximation above to yield an estimated SD. When p values were reported p < 0.05 was considered significant. Odds ratios are reported with the prefix OR, while mean differences for numerical data are reported with the prefix MD. The 95% CIs are reported in brackets.
We then imported the data yielded from Review Manager into the GRADEpro Guideline Development Tool (https://www.gradepro.org) to create standardized evidence tables. Input from the group teleconference was then used to formulate and qualitatively weight the factors affecting the recommendation.
Results
Literature Search
The initial literature search was performed with the MeSH terms as indicated in Supplemental Digital Content 1, Appendix 1, http://links.lww.com/TA/B475. Results of the search are diagrammed in Figure 1. This search yielded a total of 68 references. Forty-nine additional records were obtained from secondary searches of the references of these articles. After removing duplicates, 72 references remained. Nine reviews, four descriptive studies, and two articles that described protocols for future RCTs were excluded after screening, leaving 57 references. Two previous meta-analyses and one secondary analysis of an RCT were excluded. Three articles unrelated to the PICO question were excluded. Six other articles were excluded as they lacked a control group, leaving 45 articles. Of these, six were RCT or prospective observational studies[20–25] and seven were retrospective case-control studies (Table 1).[19][26–31] The trial by Deschodt et al.,[25] although not strictly randomized due to assignment by convenience, was analyzed with the RCT as the assignment was done prospectively. These two subgroups were selected for analysis as they were felt to have higher quality of evidence. Of note, one RCT included those aged 70 years and above,[21] One retrospective study included those aged 60 years and above,[29] while one did not have an age-based exclusion.[30] All other studies included those aged 65 years and above. Thirty-two other studies were not selected for analysis. These included 23 retrospective “before/after” studies without a contemporaneous control group, eight case series, and a single study predominantly focusing on costs.
Critical Outcomes
Inpatient/30-Day Mortality
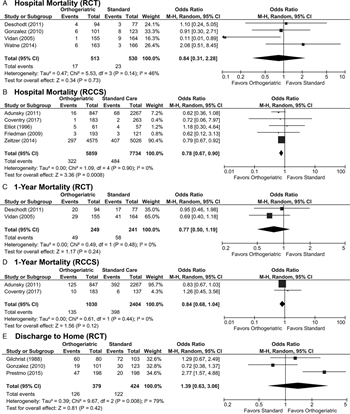
Figure 2: These forest plots indicate data for critical non-functional outcomes. (A) Odds ratio of hospital mortality in RCT are not different between groups. (B) Odds ratio of hospital mortality in RCCS are lower with OG care. (C) Odds ratio of 1-year mortality in RCT are not different between groups. (D) Odds ratio of 1-year mortality in RCCS are not different between groups. (E) Odds ratio of discharge to home are not different between groups.
For our evaluation, hospital and 30-day mortality were considered interchangeable. Four RCT evaluated hospital mortality.[20][22][24][25] Mortality ranged from less than 1% to almost 6% in the OG group and between 1.8% and 6.5% in the standard treatment group. There was a wide variation among the four studies as demonstrated by the I[2] value of 46%. Overall, the OR for mortality was not significant (OR, 0.84 [0.31, 2.28], Fig. 2A).
Five RCCS studies evaluated hospital or 30-day mortality.[19][26][28][29][31] The studies by Adunsky et al.[26] and Zeltzer et al.[31] both evaluated 30-day mortality, while the other three studies evaluated inpatient mortality. The study by Zeltzer et al. was a large database study of 37 hospitals, and thus had a larger sample size than most of the other studies. It received 90.4% of the weight in the analysis, followed by the study by Adunsky et al., which had a weight of 7.2%. Mortality rates ranged from less than 1% to 6.5% in the OG and from less than 1% to 8.1% in the standard care group, with low heterogeneity and an OR favoring OG treatment (OR, 0.78 [0.67, 0.90], Fig. 2B).
One-Year Mortality
There were two RCT that evaluated 1-year mortality.[24][25] Mortality ranged from 19% to 21% in the OG group and from 22% to 26% in the standard treatment group with little heterogeneity between studies, but the OR for mortality was not significant (OR, 0.77 [0.50, 1.19], Fig. 2C).
Among RCCS, there were two studies that evaluated 1-year mortality.[19][26] Mortality rates ranged from 5.5% to 14.7% in the OG group and from 4.4% to 17.3% in the standard care group, with low heterogeneity. Of note, neither study on its own yielded a significant result, and the combination of the two studies also did not demonstrate a significant effect (OR, 0.84 [0.68, 1.04], Fig. 2D).
Discharge to Home
Three RCT evaluated likelihood of discharge to home.[20][21][23] The percentage of patients discharged to home ranged from 19% to 75% in the OG group and from 10% to 70% in the standard care group. There was significant heterogeneity with I[2] = 79%. Odds ratio of discharge to home was not significant between groups (OR, 1.39 [0.63, 3.06], Fig. 2E).
Functional Outcomes
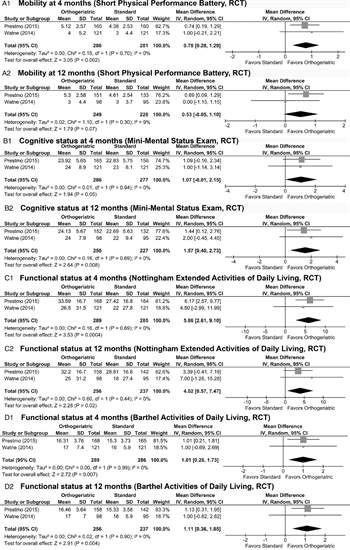
Figure 3: These forest plots indicate data for critical functional outcomes. (A) Mobility as measured by the Short Performance Physical Battery after four months was significantly higher in the OG group (1) but this was not duplicated at 12 months (2). (B) Cognitive status as measured by the Mini-Mental Status Examination after 4 months did not demonstrate a difference between groups (1) but was improved in the OG group after 12 months (2). (C) Functional status as measured by the Nottingham Extended Activities of Daily Living scale was significantly improved at both 4 months (1) and 12 months (2). (D) Functional status as measured by the Barthel Activities of Daily Living scale was significantly improved at both 4 months (1) and 12 months (2).
Two RCT evaluated functional outcomes at 4 and 12 months after injury.[21][22] The mobility at these time points was tested using the Short Performance Physical Battery.[32] There was a significant improvement identified at 4 months (MD, 0.78 [0.28, 1.29], Fig. 3A1) with low heterogeneity (I[2] = 0%) but this improvement was no longer present at 12 months (MD, 0.53 [−0.05, 1.10], Fig. 3A2).
On the contrary, there was no improvement in cognitive function as evaluated by the Mini Mental Status Examination[33] at 4 months (MD, 1.07 [−0.01, 2.15], Fig. 3B1) but there was an improvement at 12 months (MD, 1.57 [0.40, 2.73], p = 0.008, Fig. 3B2). Both examinations showed low heterogeneity (I[2] = 0%).
As far as ADLs, at 4 months, both the Nottingham Extended ADL scale[34] (MD, 5.86 [2.61, 9.10], Fig. 3C1) and the Barthel ADL scales[35] (MD, 1.01 [0.28, 1.73], Fig. 3D1) showed a statistically significant benefit for the OG group with low heterogeneity (I[2] = 0%). These findings persisted at 12 months, with a further increase demonstrated in the Nottingham Extended ADL scale (MD, 4.02, [0.57, 7.47], Fig. 3C2) and a slightly smaller increase demonstrated in the Barthel ADL scale (MD, 1.11 [0.36, 1.85], Fig. 3D2).
Important Outcomes
Hospital LOS
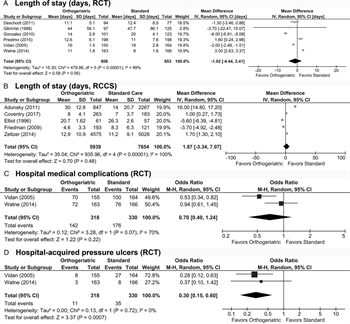
Figure 4: These forest plots indicate data for important outcomes. Hospital LOS was not different between groups in either RCT (A) or RCCS (B). (C) Hospital medical complications were not different between groups overall in RCT. (D) Odds of hospital-acquired pressure ulcer were significantly reduced in the OG group as demonstrated in RCT.
All six RCT evaluated hospital LOS.[20–25] Mean LOS ranged from 11.0 to 44.0 days in the OG group and 8.0 to 47.7 days in the standard care group, with a high degree of heterogeneity (I[2] = 99%). There was no significant difference in LOS (MD −1.02 days [−4.44, 2.41], Fig. 4A).
Five RCCS evaluated LOS.[19][26][28][29][31] Mean LOS ranged from 8.0 to 30.0 days in the OG group and 7.0 to 26.3 days in the standard care group, with an even higher degree of heterogeneity (I[2] = 100%). There was no significant difference in LOS (MD, 1.87 days [−3.34, 7.07], p = 0.48, Fig. 4B).
In-Hospital Medical Complications
Two RCT evaluated medical complications that occurred during the patient's index hospitalization.[22][24] The study by Vidan et al.[24] evaluated confusion, pressure ulcers, heart failure, pneumonia, deep venous thrombosis and pulmonary embolism, myocardial infarction, and cardiac arrhythmia. The study by Watne et al.[22] evaluated cardiac complications, cerebral complications, thromboembolic complications, pulmonary complications, renal failure, urinary tract infections, pressure ulcers, and urinary tract infections. Both articles addressed pressure ulcers, and there were fewer pressure ulcers in the Vidan study (5.2% vs. 16.9%, p = 0.001). Thus, we compared the overall rates of medical complications and the pressure ulcer rates separately. For medical complications, there was high heterogeneity (I[2] = 70%), but neither group was favored (OR, 0.70 [0.40, 1.24], Fig. 4C). For pressure ulcers, there was low heterogeneity, and the OG group was clearly favored (OR, 0.30 [0.15, 0.60], Fig. 4D).
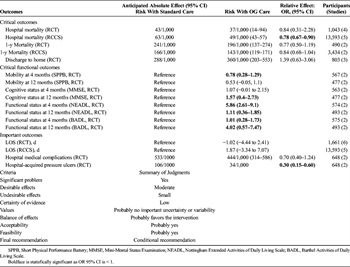
Grading the Evidence
When evaluating the quality of evidence, the authors utilized a consensus-building approach in which the articles were discussed with relationship to key attributes utilized in the GRADE methodology: study limitations, inconsistency of results, indirectness of evidence, imprecision, and reporting bias.[16] The resulting evidence table is documented in Table 2 with an additional evidence profile in Supplemental Digital Content 2, Appendix 2, http://links.lww.com/TA/B476.
For the critical outcome of mortality, inconsistency was an issue, although not serious, for the mortality outcomes. Furthermore, the benefit demonstrated by the retrospective case-control studies was not validated by the randomized or prospective studies, resulting in a serious inconsistency. Overall, a reliable estimate of effect could not be obtained. For the critical outcome of discharge to home, inconsistency was again an issue with wide disparities in the pattern of patient discharges, ultimately contributing to the lack of treatment effect demonstrated.
For the critical functional outcomes, a consistent improvement in ADL's was demonstrated both at 4 and 12 months postinjury in the OG group, while an improvement in physical performance that was demonstrated at 4 months was no longer present at 1 year and a cognitive benefit was demonstrated at 1 year but not at 4 months. The improvement in functional status is likely the strongest evidence among the available studies in terms of treatment effect, and the conclusions are quite consistent. It is worth noting that OG was not shown to be superior in two of the three critical outcomes, which played a role in downgrading our recommendation from a strong recommendation.
For the important outcome of in-hospital medical complications, a consistent treatment effect was demonstrated regarding pressure ulcers, but not to any other medical complications. There is an element of imprecision among the measurement of medical complications in general, likely contributing to the improved ability to detect a treatment effect for a single complication—pressure ulcers. For the important outcome of hospital LOS, the data was plagued again by inconsistency, as different studies that were conducted in different practice environments and different regional care systems had different paradigms for hospital discharge.
Study limitations were also an issue. Among the prospective studies, two had patients in the same ward while a third had patients split between two hospitals; neither of these is ideal for creating a controlled study environment. The retrospective studies were also limited in that bed availability was frequently used to assign treatment arms, no studies were randomized, and one study included pooled data from 37 hospitals.
Given these concerns regarding the field of literature, the unanimous impression of the authors was that the quality of evidence was low. The magnitude of the clinical problem was judged to be significant and the effect size was large for key outcomes. However, other factors should be considered in developing the strength of the recommendation. These include the balance between desirable and undesirable effects, with a larger balance in favor of desirable effects resulting in more appropriate use of a strong recommendation. Second, the values and preferences of the patient population should be considered when possible; although perhaps less applicable in this particular case, patients may have very strong preferences in certain areas (aggressive versus palliative treatment for malignancy, for example) that should either strengthen or weaken a recommendation. Finally, a low-cost intervention should be more likely to elicit a strong recommendation than a high-cost recommendation.[36]
In the case of OG versus standard care for elderly patients with hip fracture, the aggregate of the desirable effects based on the outcomes enumerated above was felt to be moderate; there was no definitive mortality benefit elicited, which surely would have prompted a strong recommendation. On the other hand, there were significant functional outcomes elicited that were favorable in the OG group. Furthermore, undesirable effects were minimal at best; in no areas did the OG treatment arm fall short. As far as the critical outcomes, there was no probable residual uncertainty or variability. Thus, it was felt by the panel that the balance of effect based on the evidence available likely favors the intervention, and that this was a feasible intervention and probably acceptable to key stakeholders. The nature of patient and family preferences as far as geriatric consultation has not been studied, but elderly patients with hip fracture have a strong preference to being discharged home and to achieve improvements in mobility, even at the cost of moderate pain.[37][38] The results from this work do not support an improvement in likelihood of discharge to home with OG treatment, but do support improvement in mobility. Thus, it stands to reason that most patients and families might view a partnership between surgeons and medical specialists favorably.
However, there are other considerations in play here. In the United States, there are fewer than half of the geriatricians needed to care for the expected number of elderly patients, not even accounting for the dramatic increase in the elderly population; dedicated geriatric wards are an even more scarce resource.[39] In many care environments, therefore, it may not be possible to provide board-certified geriatricians, even from the standpoint of solely fulfilling the need for consultation services. Thus, hospitals may have to provide alternatives in the form of multidisciplinary care team or rely on practitioners trained in internal medicine, medicine/pediatrics, or family medicine; physician extenders and telemedicine may even be an option.[39] Another option is to focus scarce geriatric resources on targeted patients.[40]
Finally, there is the issue of cost. Multiple studies have demonstrated that geriatric consultations or geriatric comanagement have the potential to reduce costs.[30][40] However, hospital systems faced with numerous financial pressures may not have the resources to invest in additional geriatric resources, even if they will save money in the long run.
With these various considerations in mind, as well as the quality of the evidence, the authors felt that an unconditional recommendation for OG consultation would be essentially declaring this practice equivalent to the standard of care, and might disadvantage many hospital systems that are unable to provide this resource due to financial constraints. The authors feel that, by leveraging a conditional recommendation nuanced based on the evidence-based benefits of OG consultation, the practitioners working in this area can innovate with regard to the best way to translate the benefits of OG care to the greatest number of patients.
Recommendation
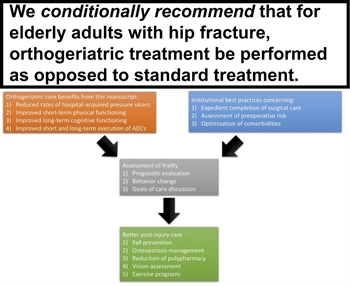
Figure 5: This diagram outlines the final recommendation of the practice management guideline (top). In addition, a putative pathway of care is also illustrated integrating recommendations in this article (orange box) with other current recommendations and best practices (blue box). This in turn results in a postacute care evaluation (gray box) that sets the foundation for future prevention measures (green box).
We conditionally recommend that for elderly adults (ages 65+) with isolated hip fracture after GLF, OG consultation be performed to reduce the rates of hospital-acquired pressure ulcers and improve short-term physical functioning, long-term cognitive functioning, and short- and long-term execution of ADL's (Fig. 5).
Discussion
Literature supporting geriatric consultation services in the acute hospital setting is not novel. A 1987 report described 113 patients, 75 years and older, who were followed up for 1 year. Patients with geriatric consultants were discharged on fewer medications, had improved mental status, and had lower short-term mortality.[39][41] Improvement in ADLs has also been shown.[39][42] However, meta-analyses have yielded discordant results.[43][44] Literature has also been inconsistent with respect to endorsing dedicated Acute Care of Elders (ACE) units, with some studies describing improved functional status, decreased LOS, and fewer readmissions while others found ACE units unnecessary and inefficient.[39] It was not until 2012, however, that data resulting from a trauma-specific ACE would be published by Mangram and colleagues, indicating decrease in emergency department, ICU, and hospital LOS, and reduced rates of mortality and infectious complications in patients older than 60 years cared for by a dedicated geriatric trauma team in a specific geriatric trauma unit.[45]
Available evidence regarding multidisciplinary treatment of isolated hip fracture patients conveys some important lessons. Firstly, mortality is not the sole critical outcome that should guide our care. Rather, when mortality improvement cannot be demonstrated, quality-of-life benefits may endorse OG care. Reduced medical complications and increased short-term mobility, long-term cognitive function, and short- and long-term functional independence in elderly adults with hip fracture endorse the OG treatment model.
Summary of this evidence may be cautiously applied beyond cases of isolated hip fracture in geriatric trauma. In the year 2025, fragility fractures are expected to number more than three million in the United States.[8] After a 2004 Surgeon General report revealed that only one out of five fragility fracture patients would receive treatment after a fracture, emphasis on secondary prevention programs for osteoporosis (interventions after a fracture) to decrease the rate of fracture recurrence is needed.[8][46] Establishing care of the fragility fracture patient under the OG model might be the first step in secondary osteoporosis prevention that has the potential to lower the risk of future fractures by 22% and save US $3.4 million annually.[47]
Once a patient suffers a fragility fracture and is hospitalized, that patient is at increased risk for additional falls.[48][49] Benefits of the OG care model enable additional emphasis on prevention via medication assessment, balance and vision assessments, and implementation of exercise programs that have been shown to reduce falls by as much as 35% in the highest-risk geriatric patients.[50]
Although a fragility fracture may be the first step in an accelerated functional decline of geriatric trauma patients, under the OG model it may also be an opportunity for improved quality of life. This could result from an evaluation of frailty.[51] Discussions between medical specialists or geriatricians and patients might prompt behavioral change, leading to improved strength or balance. Or, the quality-of-life improvement may result from geriatrician-patient or palliative care-directed conversations about prognosis, thereby improving discussions regarding short- and long-term goals of care. Studies have shown that older patients want to be thoroughly informed by their physicians regarding prognosis.[52]
Using these Guidelines in Clinical Practice
This is the first practice management guideline (PMG) using GRADE methodology to address the issue of OG treatment for elderly adults. Through a detailed analysis of the evidence, the authors offer a conditional recommendation that OG treatment may be beneficial due to the improvements measured in the rates of hospital-acquired pressure ulcers as well as improvements in short-term physical performance, long-term cognitive performance, and short- and long-term execution of ADLs. The authors would seek to encourage cautious, but broader, implementation of geriatric consultation among elderly trauma patients. This implementation may take the form of an integrated care pathway that melds the recommendations of this article with other institutional best practices to optimize in-hospital care. This, in turn, is followed by a detailed discharge assessment that sets the foundation for secondary prevention interventions and even further discussion of goals of care (Fig. 5). Such pathways, if implemented, should be carefully studied with a focus on functional outcomes, delirium, and quality of life, moving definitively beyond the mortality paradigm.
Future Investigations
This PMG sets the stage for a set of future guidelines that, collectively, could revolutionize the care of geriatric trauma patients (Fig. 5). A comparable bundle would be the ABCDEF bundle in critical care.[53] It is not accidental that this bundle, emphasizing adequate pain control, delirium prevention, early mobility, and communication with the patient and family, is chosen as an example; many of these elements are critical for geriatric trauma patients and can help assure best possible outcomes. Such a bundle would integrate the benefits outlined in this PMG and others as well as a risk assessment based on frailty. This assessment could be relatively rapid but still identify high-risk patients who would benefit from additional resources during and after hospitalization[51][54] including dedicated fall prevention training aimed at restoring confidence and improving mobility[46][55] and treatment of risk factors, including osteoporosis and polypharmacy.[56][57] High-quality randomized trials conducted at high-volume centers or even through a multicenter mechanism can identify additional elements of care that can improve outcome.
Conclusion
The authors conditionally recommend that an OG care model be used for elderly adults with hip fracture to reduce in-hospital rates of pressure ulcers and improve short-term physical functioning, long-term cognitive functioning, and short- and long-term execution of ADLs. Orthogeriatric care for elderly adults with hip fractures can be part of a gradually expanding multidisciplinary paradigm, perhaps integrating a dedicated service or unit and specialized resources to care for this challenging population.
Authorship
K.M. participated in the data analysis, formulation of recommendations, article writing, critical review. S.E.B. participated in the article writing, critical review. R.D.B. participated in the formulation of recommendations, critical review. J.J.C. participated in the formulation of recommendations, critical review. F.H. participated in the critical review. B.R.H.R. participated in the formulation of recommendations, critical review. M.L.C. participated in the formulation of recommendations, critical review, literature search.
Disclosure
The authors declare no funding or no conflicts of interest.
References
- Halaweish I, Alam HB. Changing demographics of the American population. Surg Clin North Am. 2015;95(1):1–10. PubMed | CrossRef
- Joseph B, Pandit V, Zangbar B, Kulvatunyou N, Hashmi A, Green DJ, O'Keeffe T, Tang A, Vercruysse G, Fain MJ, Friese RS, Rhee P. Superiority of frailty over age in predicting outcomes among geriatric trauma patients: a prospective analysis. JAMA Surg. 2014;149(8):766–772. View Full Text | PubMed | CrossRef
- Brooks SE, Peetz AB. Evidence-based careof geriatric trauma patients. Surg Clin North Am. 2017;97(5):1157–1174. PubMed | CrossRef
- Weir S, Salkever DS, Rivara FP, Jurkovich GJ, Nathens AB, MacKenzie EJ. One-year treatment costs of trauma carein the USA. Expert Rev Pharmacoecon Outcomes Res. 2010;10(2):187–197. View Full Text | PubMed | CrossRef
- Gupta S, Perry JA, Kozar R. Transitions of carein geriatric medicine. Clin Geriatr Med. 2019;35(1):45–52. PubMed | CrossRef
- Labib N, Nouh T, Winocour S, Deckelbaum D, Banici L, Fata P, Razek T, Khwaja K. Severely injured geriatric population: morbidity, mortality, and risk factors. J Trauma. 2011;71(6):1908–1914. View Full Text | PubMed | CrossRef
- Spaniolas K, Cheng JD, Gestring ML, Sangosanya A, Stassen NA, Bankey PE. Ground level falls are associated withsignificant mortality in elderlypatients. J Trauma. 2010;69(4):821–825.View Full Text | PubMed | CrossRef
- Greenstein AS, Gorczyca JT. Orthopedic surgery and the geriatric patient. Clin Geriatr Med. 2019;35(1):65–92. PubMed | CrossRef
- Shiga T, Wajima Z, Ohe Y. Le délai opératoire est-il associé à une mortalité accrue chez les patients atteints d'une fracture de la hanche? Synthèse systématique, méta-analyse et méta-régression. Can J Anesth/J Can Anesth. 2008;55(3):146–154.
- Maxwell CA, Miller RS, Dietrich MS, Mion LC, Minnick A. The aging of America: a comprehensive look at over 25,000 geriatric trauma admissions to United States hospitals. Am Surg. 2015;81(6):630–636. PubMed
- Bolding DJ, Corman E. Falls in the geriatric patient. Clin Geriatr Med. 2019;35(1):115–126. PubMed | CrossRef
- Kammerlander C, Roth T, Friedman SM, Suhm N, Luger TJ, Kammerlander-Knauer U, Krappinger D, Blauth M. Ortho-geriatric service—a literature review comparing different models. Osteoporos Int. 2010;21(Suppl 4):S637–S646. PubMed
- Handoll HH, Cameron ID, Mak JC, Finnegan TP. Multidisciplinary rehabilitation for older people withhip fractures. Cochrane Bone, Joint and Muscle Trauma Group, editor. Cochrane Database Syst Rev. 2009;15(1):29.
- Cameron ID, Handoll HH, Finnegan TP, Madhok R, Langhorne P. Co-ordinated multidisciplinary approaches for inpatient rehabilitation of older patients withproximal femoral fractures. Cochrane Bone, Joint and Muscle Trauma Group, editor Cochrane Database of Systematic Reviews. 2009;15(1):29.
- Calland JF, Ingraham AM, Martin N, Marshall GT, Schulman CI, Stapleton T, Barraco RD. Evaluation and management of geriatric trauma. J Trauma Acute CareSurg. 2012;73:S345–S350.
- Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, Dahm P, Eastern Association for the Surgery of Trauma Practice Management Guidelines Ad Hoc Committee. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute CareSurg. 2012;73(5 Suppl 4):S283–S287.
- Barili F, Parolari A, Kappetein PA, Freemantle N. Statistical primer: heterogeneity, random- or fixed-effects model analyses? Interact Cardiovasc Thorac Surg. 2018;27(3):317–321. PubMed
- Delgado-Rodríguez M, Sillero-Arenas M. Systematic review and meta-analysis. Med Intensiva. 2018;42(7):444–453. PubMed | CrossRef
- Coventry LS, Nguyen A, Karahalios A, Roshan-Zamir S, Tran P. Comparison of 3 different perioperative caremodels for patients withhip fractures within 1 health service. Geriatr Orthop Surg Rehabil. 2017;8(2):87–93. View Full Text | PubMed | CrossRef
- González-Montalvo JI, Alarcón T, Mauleón JL, Gil-Garay E, Gotor P, Martín-Vega A. The orthogeriatricunit for acute patients: a new model of carethat improves efficiency in the management of patients with hip fracture. Hip Int. 2010;20(2):229–235. View Full Text | PubMed | CrossRef
- Prestmo A, Hagen G, Sletvold O, Helbostad JL, Thingstad P, Taraldsen K, Lydersen S, Halsteinli V, Saltnes T, Lamb SE, Johnsen LG, Saltvedt I. Comprehensive geriatric carefor patients withhip fractures: a prospective, randomised, controlled trial. Lancet. 2015;385(9978):1623–1633. PubMed | CrossRef
- Watne LO, Torbergsen AC, Conroy S, Engedal K, Frihagen F, Hjorthaug GA, Juliebo V, Raeder J, Saltvedt I, Skovlund E, Wyller TB. The effect of a pre- and postoperative orthogeriatricservice on cognitive function in patients withhip fracture: randomized controlled trial (Oslo Orthogeriatric trial). BMC Med. 2014;12:63.
- Gilchrist WJ, Newman RJ, Hamblen DL, Williams BO. Prospective randomised study of an orthopaedic geriatric inpatient service. BMJ. 1988;297(6656):1116–1118. PubMed | CrossRef
- Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476–1482. View Full Text | PubMed | CrossRef
- Deschodt M, Braes T, Broos P, Sermon A, Boonen S, Flamaing J, Milisen K. Effect of an inpatient geriatric consultation team on functional outcome, mortality, institutionalization, and readmission rate in older adultswithhip fracture: a controlled trial. J Am Geriatr Soc. 2011;59(7):1299–1308. View Full Text | PubMed | CrossRef
- Adunsky A, Lerner-Geva L, Blumstein T, Boyko V, Mizrahi E, Arad M. Improved survival of hip fracture patients treated within a comprehensive geriatric hip fracture unit, compared withstandardof care treatment. J Am Med Dir Assoc. 2011;12(6):439–444. PubMed | CrossRef
- Adunsky A, Lusky A, Arad M, Heruti RJ. A comparative study of rehabilitation outcomes of elderlyhip fracture patients: the advantage of a comprehensive orthogeriatricapproach. J Gerontol A Biol Sci Med Sci. 2003;58(6):542–547. PubMed
- Elliot JR, Wilkinson TJ, Hanger HC, Gilchrist NL, Sainsbury R, Shamy S, Rothwell A. The added effectiveness of early geriatrician involvement on acute orthopaedic wards to orthogeriatricrehabilitation. N Z Med J. 1996;109(1017):72–73. PubMed
- Friedman SM, Mendelson DA, Bingham KW, Kates SL. Impact of a comanaged geriatric fracture Center on short-term hip fracture outcomes. Arch Intern Med. 2009;169(18):1712–1717. View Full Text | PubMed | CrossRef
- Ginsberg G, Adunsky A, Rasooly I. A cost-utility analysis of a comprehensive orthogeriatriccarefor hip fracture patients, compared with standard of care treatment. Hip Int. 2013;23(6):570–575. View Full Text | PubMed | CrossRef
- Zeltzer J, Mitchell RJ, Toson B, Harris IA, Ahmad L, Close J. Orthogeriatricservices associated withlower 30-day mortality for older patients who undergo surgery for hip fracture. Med J Aust. 2014;201(7):409–411. View Full Text | PubMed | CrossRef
- Treacy D, Hassett L. The short physical performance battery. J Physiother Korea Institute of Oriental Medicine. 2018;64(1):61.
- Kopecek M, Bezdicek O, Sulc Z, Lukavsky J, Stepankova H. Montreal cognitive assessment and mini-mental state examination reliable change indices in healthy older adults. Int J Geriatr Psychiatry. 2017;32(8):868–875. View Full Text | PubMed | CrossRef
- Wu CY, Chuang LL, Lin KC, Horng YS. Responsiveness and validity of two outcome measures of instrumental activities of daily living in stroke survivors receiving rehabilitative therapies. Clin Rehabil. 2011;25(2):175–183. View Full Text | PubMed | CrossRef
- Orive M, Aguirre U, García-Gutiérrez S, Las Hayas C, Bilbao A, González N, Zabala J, Navarro G, Quintana JM. Changes in health-related quality of life and activities of daily living after hip fracture because of a fall in elderlypatients: a prospective cohort study. Int J Clin Pract. 2015;69(4):491–500. View Full Text | PubMed | CrossRef
- Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, Schünemann HJ, GRADE Working Group. Going from evidence to recommendations. BMJ. 2008;336(7652):1049–1051. View Full Text | PubMed | CrossRef
- Robinson SM, Ní Bhuachalla B, Ní Mhaille B, Cotter PE, O'Connor M, O'Keeffe ST. Home, please: a conjoint analysis of patient preferences after a bad hip fracture. Geriatr Gerontol Int. 2015;15(10):1165–1170.
- Milte R, Ratcliffe J, Miller M, Whitehead C, Cameron ID, Crotty M. What are frail older people prepared to endure to achieve improved mobility following hip fracture? A discrete choice experiment. J Rehabil Med. 2013;45(1):81–86. PubMed | CrossRef
- Sucher JF, Mangram AJ, Dzandu JK. Utilization of geriatric consultation and team-based care. Clin Geriatr Med. 2019;35(1):27–33. PubMed | CrossRef
- Duran SF, Mazzurco L, Palmer RM. Trauma consults by geriatricians: looking into the black box. Gerontol Geriatr Med. 2018;4(4): 2333721418817668.
- Hogan DB, Fox RA, Badley BW, Mann OE. Effect of a geriatric consultation service on management of patients in an acute carehospital. CMAJ. 1987;136(7):713–717. PubMed
- McVey LJ, Becker PM, Saltz CC, Feussner JR, Cohen HJ. Effect of a geriatric consultation team on functional status of elderlyhospitalized patients. A randomized, controlled clinical trial. Ann Intern Med. 1989;110(1):79–84. PubMed | CrossRef
- Deschodt M, Flamaing J, Haentjens P, Boonen S, Milisen K. Impact of geriatric consultation teams on clinical outcome in acute hospitals: a systematic review and meta-analysis. BMC Med. 2013;11:48.
- Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. 1993;342(8878):1032–1036. View Full Text | PubMed | CrossRef
- Mangram AJ, Mitchell CD, Shifflette VK, Lorenzo M, Truitt MS, Goel A, Lyons MA, Nichols DJ, Dunn EL. Geriatric trauma service: a one-year experience. J Trauma Acute CareSurg. 2012;72(1):119–122.
- Ganda K, Puech M, Chen JS, Speerin R, Bleasel J, Center JR, Eisman JA, March L, Seibel MJ. Models of carefor the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int. 2013;24(2):393–406. PubMed | CrossRef
- Edwards BJ, Bunta AD, Simonelli C, Bolander M, Fitzpatrick LA. Prior fracturesare common in patients withsubsequent hip fractures. Clin Orthop Relat Res. 2007;461:226–230. View Full Text | PubMed
- Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21(5):658–668. View Full Text | PubMed | CrossRef
- Deandrea S, Bravi F, Turati F, Lucenteforte E, La Vecchia C, Negri E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta-analysis. Arch Gerontol Geriatr. 2013;56(3):407–415. PubMed | CrossRef
- Campbell AJ, Robertson MC. Comprehensive approach to fall prevention on a national level: new Zealand. Clin Geriatr Med. 2010;26(4):719–731. PubMed | CrossRef
- Maxwell CA, Patel MB, Suarez-Rodriguez LC, Miller RS. Frailty and prognostication in geriatric surgery and trauma. Clin Geriatr Med. 2019;35(1):13–26. PubMed | CrossRef
- Bernacki RE, Block SD, American College of Physicians High Value CareTask Force. Communication about serious illness caregoals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994–2003. View Full Text | PubMed | CrossRef
- Ely EW. The ABCDEF bundle: science and philosophy of how ICU liberation serves patients and families. Crit CareMed. 2017;45(2):321–330. View Full Text | PubMed | CrossRef
- Maxwell CA, Dietrich MS, Miller RS. The FRAIL questionnaire: a useful tool for bedside screening of geriatric trauma patients. J Trauma Nurs. 2018;25(4):242–247. View Full Text | PubMed | CrossRef
- Kampe K, Kohler M, Albrecht D, Becker C, Hautzinger M, Lindemann U, Pfeiffer K. Hip and pelvic fracture patients withfear of falling: development and description of the “step by step” treatment protocol. Clin Rehabil. 2017;31(5):571–581. View Full Text | PubMed | CrossRef
- Axelsson KF, Wallander M, Johansson H, Lundh D, Lorentzon M. Hip fracture risk and safety withalendronate treatment in the oldest-old. J Intern Med. 2017;282(6):546–559. PubMed | CrossRef
- Rizk P, Morris W, Oladeji P, Huo M. Review of postoperative delirium in geriatric patients undergoing hip surgery. Geriatr Orthop Surg Rehabil. 2016;7(2):100–105. View Full Text | PubMed | CrossRef
Keywords:
Geriatric; hip fracture; orthogeriatric; elderly; functional outcome; mortality
Supplemental Digital Content
- TA_2019_08_19_MUKHERJEE_JT-D-19-09726_SDC1.docx; [Word] (12 KB)
- TA_2019_08_19_MUKHERJEE_JT-D-19-09726_SDC2.docx; [Word] (50 KB)
© 2020 Lippincott Williams & Wilkins, Inc.