Non-Surgical Management and Analgesia Strategies for Older Adults with Multiple Rib Fractures: a Systematic Review, Meta-Analysis, and Practice Management Guideline
Published 2022
Citation: Journal of Trauma and Acute Care Surgery: November 15, 2022 - Volume - Issue 10.1097
Authors
Kaushik Mukherjee, MD, MSCI1, Sebastian D. Schubl, MD2, Gail Tominaga, MD3, Sarah Cantrell, MLIS, AHIP4, Brian Kim, MD5, Krista L. Haines, MA, DO4, Krista L. Kaups, MD6, Robert Barraco, MD7, Kristan Staudenmayer, MD8, Lisa M. Knowlton, MD, MPH8, Adam M. Shiroff, MD9, Zachary M. Bauman, DO10, Steven E. Brooks, MD11, Haytham Kaafarani, MD12, Marie Crandall, MD, MPH13, Raminder Nirula, MD14, Suresh K. Agarwal Jr., MD4, John J. Como, MD15, Elliott R. Haut, MD16, George Kasotakis, MD, MPH4
1Loma Linda University Medical Center, Loma Linda, CA
2University of California Irvine Medical Center, Irvine, CA
3Scripps Mercy Health, San Diego, CA
4Duke University Medical Center, Durham, NC
5The Mayo Clinic, Rochester, MN
6University of California San Francisco-Fresno, Fresno, CA
7Lehigh Valley Health Network, Allentown, PA
8Stanford University Medical Center, Palo Alto, CA
9University of Pennsylvania Medical Center, Philadelphia, PA
10University of Nebraska Medical Center, Omaha, NE
11Texas Tech University Health Sciences Center, Lubbock, TX
12Massachusetts General Hospital, Boston, MA
13University of Florida College of Medicine, Jacksonville, FL
14University of Utah Medical Center, Salt Lake City, UT
15MetroHealth Cleveland Medical Center, Cleveland, OH
16Johns Hopkins Medical Center, Baltimore, MD
Corresponding Author
George Kasotakis, MD, MPH
Assistant Professor of Surgery
Division of Trauma and Critical Care Surgery
Department of Surgery
Duke University School of Medicine
Duke University Medical Center
DUMC 2837
2301 Erwin Road
Durham, NC 27710
Phone: 919-681-4925
Fax: 919-668-4369
Email: george.kasotakis@duke.edu
Authorship
All authors: Development of PICO questions, voting on outcomes, voting on recommendations, critical review and editing of manuscript
KM: meta-analysis, formulation of recommendations with group supervision, writing manuscript
SDS: writing manuscript, review of articles
GT: writing manuscript, review of articles
BK, KH, KK, RB, KS, AMS, ZB: review of articles
HK, MC, SA, JJC, EPH, GK: Development of recommendations, critical overview of KM’s meta-analysis and formulation of recommendations
Acknowledgment: The authors would also like to thank the Eastern Association for the Surgery of Trauma, the Chest Wall Injury Society, and the American Association for the Surgery of Trauma Patient Assessment Committee.
Disclosures: SDS and ZMB are educational consultants for Zimmer Biomet Inc. AMS is an education consultant for Globus Medical, Inc. and for Synthes USA Products LLC.
Funding: No external funding was available for this project.
Introduction
Rib fractures occur in 10% of trauma patients and are associated with lung contusions, hemopneumothorax, and cardiac injuries; they are also common in the elderly.[1] Complications of multiple rib fractures, including increased pneumonia rates, ventilator dependence, ICU and hospital length of stay (LOS), and mortality in adults aged ≥65 years compared to younger patients with similar injury severity.[2][3] Mortality risk increases by 19% and nosocomial infection risk increased by 29% with each additional rib fracture.[2] Due to continuous chest wall motion during respiration, ribs require significantly longer to heal than fractures that can be immobilized; disability prevalence approaches 40% six months after injury.[4] Due to the impact of rib fractures on outcomes in older adults, we developed a practice management guideline (PMG) for this population.[3][5] Specifically, selection criteria for intensive care unit (ICU) admission, the use of locoregional anesthesia and non-invasive positive pressure ventilation (NIPPV) are not well-defined. Similarly, the utility of incentive spirometry (IS) and both single as well as multimodal pain medications are unclear in older adults. Recovery of patients from chest wall injury is prolonged, with low-severity injuries leading to chronic pain syndromes and high-severity injuries impacting quality of life and functional independence.[5] We performed a systematic review and meta-analysis concerning nonoperative management of chest wall injury.
Methods
Objectives
A joint working group of Eastern Association for the Surgery of Trauma (EAST), Chest Wall Injury Society (CWIS), and American Association for the Surgery of Trauma (AAST) Patient Assessment Committee members evaluated nonoperative interventions for adults ≥65 presenting with multiple rib fractures. GRADE methodology was used as outlined by Kerwin et.al.[6]
Selection of Outcomes and PICO Questions
Candidate outcomes for each PIC stem were voted upon by each author using a 1-9 scale. Outcomes scoring 7-9 were considered critical and included in our analysis. The outcomes and scores are summarized in Supplementary Table 1. The following PIC and Outcome (PICO) questions were formulated prior to literature search: #1: In adults aged ≥65 years old with ≥3 rib fractures (P), should admission to an ICU setting (I) versus admission to a non-ICU setting (C) take place to reduce pneumonia, need for intubation, ventilator days, or mortality (O)?
#2: In adults aged ≥65 years old with rib fractures (P), should routine use of IS (I) versus no routine use of IS (C), be performed to reduce pneumonia, need for intubation, or mortality (O)?
#3: In adults aged ≥65 years old with rib fractures and acute hypoxic respiratory failure refractory to nasal cannula and face mask (P), should NIPPV (high-flow nasal cannula [HFNC], bi-level positive airway pressure [BiPAP], continuous positive airway pressure [CPAP]) (I) versus endotracheal intubation (C) be utilized to reduce pneumonia, need for intubation, ventilator days, or mortality (O)?
#4: In adults aged ≥65 years old with ≥3 rib fractures and dyspnea or refractory pain (P), should ketamine infusion plus structured multi-modal pain therapy per institutional protocol (I) versus structured multi-modal pain therapy per institutional protocol alone (C) be performed to reduce pain, pneumonia, need for intubation, ventilator days, or mortality (O)?
#5: In adults aged ≥65 years old with ≥3 rib fractures and dyspnea or refractory pain, should a thoracic epidural catheter and structured multi-modal pain therapy per institutional protocol (I) versus structured multi-modal pain therapy per institutional protocol alone (C) be performed to reduce pain, pneumonia, need for intubation, hospital length of stay, or mortality (O)?
#6: In adults aged ≥65 years old with ≥3 rib fractures and dyspnea or refractory pain, should nonepidural locoregional anesthetic (subcutaneous infusion pump or local block) and structured multi-modal pain therapy per institutional protocol (I) versus structured multi-modal pain therapy per institutional protocol alone (C) be performed to reduce pain, pneumonia, need for intubation, ventilator days, or mortality(O)?
Literature Search
This study conforms with the PRISMA guidelines and a complete checklist has been uploaded as Supplementary Table 2, along with the Abstract checklist as Supplementary Table 3. Our systematic review was registered with PROSPERO (2020-CRD42020201241). Search strategy included MEDLINE, EMBASE, Cochrane Library, and Web of Science (Supplementary Table 4). Published retrospective case-control, prospective observational, and randomized studies in English that included both patients receiving the intervention and comparator were eligible for review. Case series and reports, commentaries, animal studies, and operative technique articles were excluded. Systematic reviews were assessed to ensure their referenced primary studies were included.
Review of Abstracts and Full-text Articles
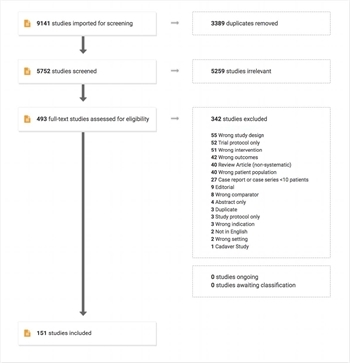
Figure 1 The PRISMA flow diagram indicates the flow of studies and their review process for the systematic review.
Titles, abstracts and full texts were reviewed independently by two team members, and any conflicts were adjudicated by a third. All reviews took place in Covidence (www.covidence.org). The list of studies (Supplementary Table 5) and PRISMA flow diagram (Figure 1) are included.
Data Extraction and Analysis
Data extraction was performed by a single author (KM) with group oversight and review to ensure accuracy. Meta-analysis was performed using RevMan 5.0 (http://revman.cochrane.org), including odds ratios (OR) and 95% confidence intervals (95%CI). Heterogeneity was calculated and quantified (I2<50% acceptable, 50-74% borderline, ≥75% high).[7]
Assessment of Quality of Evidence
Quality of evidence was assessed with GRADEPro (www.gradepro.org), considering potential risk of bias; inconsistency; indirectness; imprecision; and publication bias. Authors voted on recommendations for each PICO question, evaluating the relationship of benefits and harms, patient values and preferences, and resource utilization.[6]
Results
#1: ICU Admission
Qualitative Analysis
Six retrospective studies were included. Athanassiadi et.al. found ISS predicted morbidity and hospital length of stay but did not discuss ICU admission.[8] Bellone et.al. found that trauma score and obliged orthopnea were associated with ICU admission within the first 72 hours.[9] Blecher et.al. found that nearly 10% of patients (70/764) initially triaged to the floor required ICU admission, and that tube thoracostomy was an independent predictor of ICU need.[10] Bowman et.al. found incentive spirometry <1L, use of a walker, chest-AIS score of 3 or higher, age ≥72 years, and active smoking were independent risk factors for critical patient incidents in non-ICU patients, although there were only 7 incidents in the 150-patient study.[11] Pyke et.al. studied outcomes before and after implementation of a protocol admitting older patients with ≥3 rib fractures to the ICU. Although the recommendation was not universally followed, the post-intervention group had fewer complications, unplanned ICU admissions, and non-home discharges.[12] Shi et.al. found, conversely, when their institutional guideline for ICU admission for patients ≥65 years with ≥2 rib fractures was not followed, only 9% of patients had delayed ICU admission.[13]
These studies were retrospective with different definitions. Some studies included older patients,[11–13] while other studies included all patients. There was little discussion regarding the predetermined critical outcomes. Guidelines for ICU admission were also frequently not followed. Risk of bias was high and quality of evidence was very low. This data did not support any quantitative analysis nor any recommendations.
#2: IS
Qualitative Analysis
There were three retrospective studies and one randomized clinical trial (RCT) utilizing IS. Bakhos et.al. noted that vital capacity (actual and percent predicted), related to IS, correlated with hospital length of stay and discharge home. The study population had median age 80 years with 3.6±1.6 rib fractures. Patients with a VC<1.4L or <55% of predicted had a hospital length of stay of more than 3 days.[14] Kelley et.al. used IS to guide treatment with noninvasive positive pressure ventilation (NIPPV) as part of a chest injury protocol that reduced pneumonia, unplanned intubation, and unplanned ICU admission in 316 patients. If IS was <750mL despite the NIPPV trial, the settings were increased and additional pain medications added with consideration for ICU transfer.[15] In a retrospective review, Sadler et.al. noted that patients with IS <1L had a 3.3-fold increased risk of pulmonary complications and increased likelihood of non-home discharge. In this study, mean age was 54 years and patients had an average of four rib fractures.[16] In the 50-patient RCT, patients receiving IS were less likely to have pulmonary complications, largely defined by retained hemothorax. Pulmonary function testing was also performed and showed decreased forced vital capacity and forced expiratory volume in 1 second (FEV1) in the control group. The population in this group had a mean age of 55 and approximately 80% of patients had at least 3 rib fractures.[17]
In assessing the literature, data quality was low with one RCT and three retrospective studies, and no quantitative analysis was possible. However, other factors considered included good therapeutic tolerance, few side effects, and low cost. Thus, we recommended that IS be used to reduce overall pulmonary complications.
PICO #3: NIPPV
Qualitative Analysis
This field of literature included two RCT directly addressing the topic and a third with a different control group, as well as three retrospective studies. Carrie et.al. studied NIPPV as part of an overall blunt chest trauma protocol with 1:1 nearest neighbor matching. The use of NIPPV increased from 6% to 39% with the protocol, but no results were reported directly attributable to the use of NIPPV.[18] NIPPV was likewise part of the protocol developed by Kelley et.al., which noted a reduced rate of unplanned ICU admissions, unplanned intubations, and pneumonia; it is again unclear exactly what proportion of these outcomes is attributable to NIPPV.[15] Halub et.al. conducted a retrospective study of high flow nasal cannula (HFNC) set to 50 L/min at 50% FIO2 in patients with median age 63 and ISS 21. She reported an 18% failure rate for HFNC, with 69% of patients on HFNC never needing intubation.[19]
Hernandez et.al. performed a single-center RCT of patients with mean age in the mid-40’s, mean chest-AIS of 4, and PaO2/FIO2 ratio <200 for more than 8 hours within the first 48 hours after injury. The control arm received supplemental oxygen (≥10 L/min) by mask and the experimental arm received NIPPV with a BiPap mask set to 10-12 cm H2O IPAP and 6 cm H2O EPAP. The intubation rate was 40% in the control arm versus 12% in the NIPPV arm (p=0.02).[20] This study could not be included in quantitative analysis as the control group did not include endotracheal intubation. However, it does provide good quality evidence that NIPPV may be beneficial, and more closely approximates the practice pattern at many institutions.
The RCT by Bolliger et.al. compared patients aged > 40 years with >3 rib fractures. Patients were randomized to CPAP mask and epidural analgesia or ETT. The CPAP group demonstrated a shorter treatment course (4.5 days vs. 7.3 days, p=0.0003), shorter ICU and hospital length of stay (LOS), and a lower pneumonia rate.[21] The RCT by Gunduz et.al. compared patients in their 30’s-40’s with ≥5 consecutive rib fractures or flail chest, respiratory rate >25, O2 saturation <90% on 10L face mask, and a PaO2/FIO2 ratio <300 on ≥50% FIO2 in the ICU. 27 patients received mechanical ventilation and 25 received CPAP. The CPAP group had a lower rate of nosocomial infection and higher survival.[22] Overall quality of evidence was moderate, with some indirectness in multiple studies.
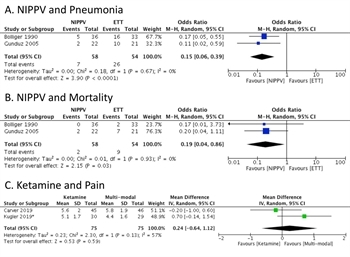
Figure 2 A. Non-invasive positive pressure ventilation (NIPPV) is associated with decreased incidence of pneumonia in two randomized controlled clinical trials. B. NIPPV is associated with decreased incidence of pneumonia in two randomized controlled clinical trials. C. Ketamine infusion is not demonstrably superior to multi-modal pain medication in two closely-related randomized controlled clinical trials. The trial by Kugler and co-authors is asterisked as it specifically included elderly patients.
Quantitative Analysis
The studies by Bolliger et.al. and Gunduz et.al. were also suitable for meta-analysis for two endpoints: pneumonia and mortality (Figure 2A-B) with a total of 58 patients in the NIPPV group and 54 in the endotracheal intubation (ETT) group. The pooled OR for pneumonia was 0.15 [0.06, 0.39] with p <0.0001 in favor of CPAP. The pooled OR for mortality was 0.19 [0.04, 0.86] with p<0.03 in favor of CPAP. The heterogeneity was 0% in both comparisons. Data from the meta-analysis was favorable with low risk of bias. However, the group felt a full recommendation for NIPPV should not be offered as all the studies had multiple exclusions, including patients with traumatic brain injury, GCS < 15, or inadequate airway protection. This was done to ensure that patients would not suffer adverse events due to aspiration or other airway events. Thus, we suggest that NIPPV be used in carefully selected patients with persistent acute hypoxic respiratory failure as defined as O2 saturation <90% on supplemental oxygen ≥10L by face mask after optimizing analgesia and ensuring that patients could protect their airway and avoid complications of airway failure. Patients whose O2 saturations do not improve above 90% on NIPPV or who have worsening of their mental status or airway compromise should then proceed to ETT.
PICO #4: Ketamine Infusion
Qualitative Analysis
The literature concerning the use of ketamine for analgesia in adults with rib fractures consists of a retrospective case-control study of 30 patients and two RCT that were organized similarly but included two differing populations. The retrospective study by Walters et.al. of ketamine infusion at 0.1 mg/kg/hr for ICU patients (mean age 59 years) with ≥1 rib fracture and ISS >15 demonstrated that numeric pain score and opioid use were lower in the ketamine group compared to the standard group with a hydromorphone patient-controlled analgesia (PCA) pump.[23] The study by Carver et.al. included adults ≤ 64 years with ≥3 rib fractures and randomized patients to ketamine at 0.15 mg/kg/hr versus normal saline. This study found no significant difference in numeric pain score or oral morphine equivalent (OME) use after 24 hours in the overall patient population, but did find a reduction in OME for patients with ISS >15 in the first 24 and 48 hours and overall.[24] A similar trial conducted by Kugler et.al. of adults ≥65 years with ≥3 rib fractures randomized patients to ketamine at 0.12 mg/kg/hr versus normal saline. Both the Kugler et.al. and Carver et.al. studies utilized an institutional thoracic pain management protocol utilizing both opioid and non-opioid medications; further details were not provided. This study found no significant effect of ketamine in the overall population but did note a decrease in OME for the ketamine group in the first 24 hours.[25]
Quantitative Analysis
The trials by Kugler et.al. and Carver et.al. were amenable for meta-analysis for numeric pain score but demonstrated no significant treatment effect (OR 0.24 [-0.64, 1.12, p = 0.59], Figure 2C above). Overall, there were other concerns with the published literature, including the lack of standardization of pain management in the control groups and the indirectness associated with the Carver et.al. trial from the exclusion of older patients. Quality of evidence was felt to be low to moderate. No recommendation for or against the use of ketamine was indicated.
PICO #5: Epidural Analgesia
Qualitative Analysis
The literature had several studies that could not be utilized for further analysis. Six studies were prospective or randomized comparisons of either different epidural techniques or epidural and locoregional techniques with no other control group.[26–31] Five studies were either case series or evaluated care bundles.[18][32–35] There were 12 retrospective studies with 3916 patients (913 with epidural catheters and 3903 without) with very low to low quality of evidence.[2][36–46] There were also 4 RCT with a total of 79 patients, half with epidurals and half without. The study by Ahmed et.al. enrolled only mechanically ventilated patients between 18-55 years with ≥3 rib fractures and a flail segment.[47] Bulger et.al. enrolled patients with ≥3 rib fractures and a mean age in the 40’s.[48] Mackersie et.al. included patients with ≥3 rib fractures, flail chest or sternal fracture, or ≥2 rib fractures and exploratory laparotomy or pulmonary contusion, with a mean patient age in the 40’s.[49] Ullman et.al. included patients with mean ages in the 40’s-50’s, multiple rib and/or pelvic fractures, and ICU admission.[50]
Among the retrospective and prospective studies, a surprisingly small number actually studied pain as an outcome. Furthermore, there was no standardization of the pain management in the control arm, with most studies using a mixture of intravenous and oral opioids and little use of non-opioid analgesics. For example, the study by Bulger et.al. indicated that 38% of patients above the age of 65 received acetaminophen and 14% received ketorolac; use of GABAergic agents was not tabulated. Essentially all older patients received some combination of intravenous and oral opioids.[2] There was one retrospective study demonstrating some improvement in numeric pain scores when epidural bupivacaine and fentanyl were compared to intravenous patient-controlled analgesia with intravenous morphine, but this was not corroborated in the single prospective study available, which compared epidural versus intravenous fentanyl administration.[44][49] An additional retrospective study which focused on delirium rates did not find a change in numeric pain scores but did note a decreased daily morphine equivalent dose after epidural catheter placement.[42] Due to the number of studies and the differences in study methodology, we proceeded directly to quantitative analysis for the other outcomes.
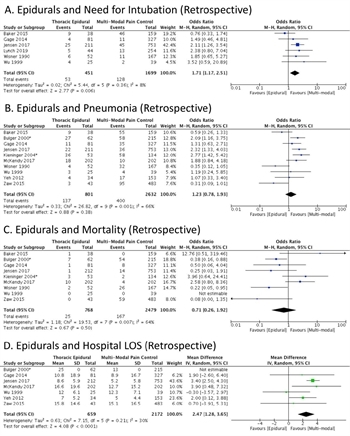
Figure 3. A. Retrospective studies evaluating the need for endotracheal intubation and mechanical ventilation with and without epidural analgesia are listed. There is a net effect that favors treatment without epidural analgesia. B. Retrospective studies are evaluated for the incidence of pneumonia with and without epidural analgesia. There is no net effect. The study by Kieninger and co-authors is asterisked as it specifically included elderly patients. C. There is no net effect on mortality with the use of epidural analgesia. The study by Kieninger and co-authors is asterisked as it specifically included elderly patients. D. Retrospectively, epidural analgesia is associated with increased hospital length of stay in days. The study by Bulger and co-authors is asterisked as it specifically included elderly patients.
Quantitative Analysis
Among retrospective studies, use of epidural analgesia was associated with increased risk of ETT (OR 1.71 [1.17-2.51, p=0.006], Figure 3A), in data with relatively low heterogeneity. There was no significant relationship between epidural use and either pneumonia (Figure 3B) or mortality (Figure 3C); data had acceptable heterogeneity (I2=30%). Epidural use was associated with a prolonged hospital LOS (2.47 days [1.28-3.65, p<0.0001, Figure 3D) and did not have a discernible effect on length of mechanical ventilation (Supplementary Fig 1A). The overall quality of evidence was very low to low.
Among prospective studies with epidurals, two studies demonstrated a non-significant trend toward reduced pneumonia (OR 0.37 [0.12- 1.15, p=0.09, Figure 4A). There was no significant effect on mortality, hospital LOS, or length of mechanical ventilation, and these data had high heterogeneity (Figure 4B-4D). Quality of evidence was moderate rather than high due to the absence of standardization among pain control regimens. 11/19 coauthors favored no recommendation for or against, four favored a suggestion for epidural use, three favored against, and one voted to recommend against. The group therefore elected to make no recommendation for or against epidural analgesia.
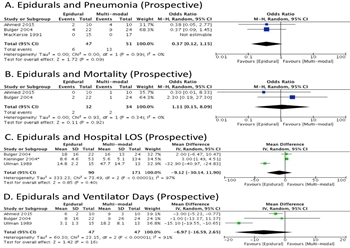
Figure 4. A. Prospective studies do not indicate a benefit for epidural analgesia with respect to pneumonia. B. Prospective studies do not indicate a benefit for epidural analgesia with respect to mortality. C. Hospital length of stay in days is not significantly different with or without epidural analgesia. The study by Kieninger and co-authors is asterisked as it specifically included elderly patients. D. Epidural analgesia does not result in a significant difference in ventilator days.
PICO #6: Non-epidural Locoregional Analgesia
Qualitative Analysis
The field of literature had several studies that could not be utilized for further analysis. Seven were case series, two of which evaluated pain before and after locoregional analgesia.[51–57] The study by Hernandez et.al. noted a decrease in numeric pain score from median of 7 before serratus anterior block to a median of 3 four hours after the block, with the type of local anesthetic at provider discretion.[56] No subsequent pain scores were obtained. Adhikary et.al. indicated that use of an erector spinae block with ropivicaine reduced pain scores from a mean of 8 (24 hours prior to block) to 5-6 in the next 24 hours.[57] Six studies were comparisons primarily between epidural and other locoregional techniques, without a standardized control group.[26][27][29–31][40] One study evaluated a care bundle, while another predominantly studied delirium and did not break down outcomes by type of locoregional analgesia.[18][42] This left two RCT. Ge et.al. studied 90 patients with mean age of 40 years and an average of 4 rib fractures and randomized patients to intravenous opioid patient controlled analgesia versus thoracic paravertebral catheters. This study reported reduced numeric pain scores and improved pulmonary function tests in the paravertebral catheter group. Visual analog pain scores at rest decreased by 1 point on day 1, 1.1 points with coughing on day 1, 0.7 points at rest on day 2, 0.6 points with coughing on day 2, 0.2 points with coughing on day 3, and 0.1 points with coughing on day 4.[58] Gabram et.al. conducted a RCT of 42 patients in their 50’s with approximately 4 rib fractures and a forced vital capacity <70% of predicted. There were baseline differences between groups; the intrapleural catheter group had fewer rib fractures and a lower initial percentage of predicted forced vital capacity. There were no significant differences in the results.[59] The overall quality of these studies was moderate due to potential allocation differences in the Gabram et.al. study and absence of non-opioid analgesics.
Quantitative Analysis
The studies performed by Gabram et.al. and Ge et.al. were amenable to meta-analysis for pneumonia (Supplementary Figure 1B). There was no difference identified, and heterogeneity was acceptable (I2=44%). 12/19 coauthors voted for no recommendation, five favored a suggestion, one voted for a recommendation, and one favored a suggestion against. The final decision was to offer no recommendation for or against.
Discussion
Grading the Evidence
For PICO #1 (ICU), the included studies were all retrospective in nature and also indirect, either assessing outcomes or a larger population than the PICO question. There were also varying inclusion criteria based on injury severity, resulting in very low quality of evidence (Supplementary Table 6). For PICO #2 (IS) there were three retrospective studies and one RCT, with overall low quality based on non-serious indirectness (one study utilized assessment of vital capacity rather than IS). For this PICO question the mean age was in the mid 50’s with at least 3 rib fractures in most patients (Supplementary Table 7). For PICO #3 (NIPPV), the quality of evidence began as very high due to the inclusion of 2 RCT’s with large effect sizes, but was downgraded one point for indirectness due to age and injury severity variance between the RCT’s and the PICO population (Supplementary Table 8). For PICO #4 (ketamine), the quality of evidence began as high due to 2 RCT’s with small effect size, but was then downgraded twice: once for risk of bias due to variations in pain management techniques in the control group and absence of non-opioid medications, and secondly for indirectness as one RCT explicitly excluded older patients (Supplementary Table 9). For PICO #5 and #6 (epidurals and other locoregional anesthesia) the quality of evidence was initially high due to RCT’s, but then downgraded three times: once for serious risk of bias due to variations in the control pain regimen between the studies, secondly for inconsistency due to heterogeneity, and finally for indirectness due to age variance between the study populations and the PICO population, resulting in a final classification of very low to low quality of evidence (Supplementary Tables 10, and 11).
Limitations
This systematic review has some limitations. Focusing on patients aged ≥65 years led to a large amount of available literature being indirect and thus lowered the quality of applicable evidence. Literature in languages other than English was excluded, while some retrospective studies were included due to the low number of high-quality RCT in this area. Variation in trial methodologies and populations occurred frequently. Finally, in any meta-analysis, the possibility of differing results and interpretations thereof in the field of literature elicited always exists, which has the potential to alter study conclusions.
Recommendations
Recommendations are summarized in Table 1.
Using these Guidelines in Clinical Practice
This PMG highlights the evidence pertaining to the nonsurgical management of patients >65 years with rib fractures. Although we cannot offer a recommendation on ICU admission for all patients above the age of 65, ICU admission should be considered in patients who also have frailty or deconditioning, hypoxemia (room air O2 sat <92%), IS <1L, more severe chest injuries (chest AIS>3), use of a walker, and smoking.[11][13][60–62]
IS and effective pain control are mainstays of management. Poor use of IS(<1L) or presence of tube thoracostomy should prompt close monitoring.[16][10] NIPPV (including HFNC and BiPAP) is suggested for selected patients aged ≥65 years with rib fractures and acute hypoxic respiratory failure refractory to nasal canula and face mask who do not require airway protection for other reasons (hemodynamic instability, TBI, somnolence, severe acidosis, etc.) and have no contraindication for face mask use (agitated or uncooperative, multiple facial fractures, abdominal distension, etc.).[20–22] NIPPV failure should result in prompt ETT.
Adequate pain control is essential in the treatment of rib fractures. Data is lacking to support the use of ketamine, locoregional blocks and epidural analgesia/anesthetics in this patient population as superior to multimodal analgesia. Of note, a previous guideline was published with a different recommendation on epidural catheters suggesting their use.[63] This conclusion resulted from two studies not included here.[64][65] One included data from a conference abstract that was not subsequently published and thus did not meet inclusion criteria for this review. The second primarily examined inflammatory markers but also noted a decrease in numeric pain scores on days 1 and 3 after catheter placement; this study could not be meta-analyzed as standard deviation was not provided. Data review did not demonstrate convincing data for the PICO questions, thus our recommendation differs from the previous one. These recommendations should not be interpreted as meaning epidural analgesia and other locoregional techniques should not be used, nor should they be interpreted as a recommendation to increase opioid use. Rather, we advocate a multifaceted pain management strategy based on multimodal analgesia and other techniques according to provider judgment and institutional resources.
Supplemental Digital Content
Supplementary Table 1: List of PICO Questions
Supplementary Table 2: PRISMA 2020 Checklist
Supplementary Table 3: PRISMA 2020 Abstract Checklist
Supplementary Table 4: Search Strategies
Supplementary Table 5: List of All Studies
Supplementary Table 6: Evidence Table for PICO #1
Supplementary Table 7: Evidence Table for PICO #2
Supplementary Table: Evidence Table for PICO #3
Supplementary Table: Evidence Table for PICO #4
Supplementary Table: Evidence Table for PICO #5
Supplementary Table: Evidence Table for PICO #6
Locoregional Anesthesia and Pneumonia (Prospective)
References
- Lafferty PM, Anavian J, Will RE, Cole PA. Operative Treatment of Chest Wall Injuries. J Bone J Surg. 2011;93:97–110.
- Bulger EM, Arneson MA, Mock CN, Jurkovich GJ. Rib fractures in the elderly. J Trauma. 2000;48:1040-7.
- Bastos R, Calhoon JH, Baisden CE. Flail Chest and Pulmonary Contusion. Sem Thorac Cardiovasc Surg. 2008;20:39–45.
- Tulay CM, Yaldiz S, Bilge A. Do we really know the duration of pain after rib fracture? Kardiochirurgia Torakochirurgia Polska Pol J Cardio-thoracic Surg. 2018;15:147–50.
- Shelat VG, Eileen S, John L, Teo LT, Vijayan A, Chiu MT. Chronic pain and its impact on quality of life following a traumatic rib fracture. Eur J Trauma Emerg S. 2012;38:451–5.
- Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, et al. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg. 2012;73:S283-7.
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. Available at: https://onlinelibrary.wiley.com/doi/full/10.1002/sim.1186, Accessed May 1, 2022.
- Athanassiadi K, Gerazounis M, Theakos N. Management of 150 flail chest injuries: analysis of risk factors affecting outcome. Eur J Cardiothoracic Surg 2004;26:373–6.
- Bellone A, Bossi I, Etteri M, Cantaluppi F, Pina P, Guanziroli M, et al. Factors Associated with ICU Admission following Blunt Chest Trauma. Can Respir J. 2016:3257846.
- Blecher GE, Biswadev M, A. CP, Mark F. Failed Emergency Department disposition to the ward of patients with thoracic injury. Inj. 2008;39:586–91.
- Bowman JA, Jurkovich GJ, Nishijima DK, Utter GH. Older Adults With Isolated Rib Fractures Do Not Require Routine Intensive Care Unit Admission. J Surg Res. 2020;245:492–9.
- Pyke OJ, Rubano JA, Vosswinkel JA, McCormack JE, Huang EC, Jawa RS. Admission of elderly blunt thoracic trauma patients directly to the intensive care unit improves outcomes. J Surg Res. 2017;219:334–40.
- Shi HH, Esquivel M, Staudenmayer KL, Spain DA. Effects of mechanism of injury and patient age on outcomes in geriatric rib fracture patients. Trauma Surg Acute Care Open. 2017;2:1-5.
- Bakhos C, O’Connor J, Kyriakides T, Abou-Nukta F, Bonadies J. Vital Capacity as a Predictor of Outcome in Elderly Patients with Rib Fractures. J Trauma. 2006;61:131–4.
- Kelley KM, Burgess J, Weireter L, Novosel TJ, Parks K, Aseuga M, et al. Early Use of a Chest Trauma Protocol in Elderly Patients with Rib Fractures Improves Pulmonary Outcomes. Am Surg. 2019;85:288–91.
- Sadler CA, Burgess JR, Dougherty KE, Collins JN. Bedside Incentive Spirometry Predicts Risk of Pulmonary Complication in Patients with Rib Fractures. Am Surg. 2019;85:1051–5.
- Sum S-K, Peng Y-C, Yin S-Y, Huang P-F, Wang Y-C, Chen T-P, et al. Using an incentive spirometer reduces pulmonary complications in patients with traumatic rib fractures: a randomized controlled trial. Trials. 2019;20:797.
- Carrie C, Stecken L, Cayrol E, Cottenceau V, Petit L, Revel P, et al. Bundle of care for blunt chest trauma patients improves analgesia but increases rates of intensive care unit admission: A retrospective case-control study. Anaesth Crit Care. 2018;37:211–5.
- Halub ME, Spilman SK, Gaunt KA, Lamb KD, Jackson JA, Oetting TW, et al. High-flow nasal cannula therapy for patients with blunt thoracic injury: A retrospective study. Can J Respir Ther. 2016;52:110–3.
- Hernandez G, Fernandez R, Lopez-Reina P, Cuena R, Pedrosa A, Ortiz R, et al. Noninvasive Ventilation Reduces Intubation in Chest Trauma-Related Hypoxemia A Randomized Clinical Trial. Chest. 2010;137:74–80.
- Bolliger CT, Eeden SFV. Treatment of Multiple Rib Fractures Randomized Controlled Trial Comparing Ventilatory with Nonventilatory Management. Chest. 1990;97:943–8.
- Gunduz M, Unlugenc H, Ozalevli M, Inanoglu K, Akman H. A comparative study of continuous positive airway pressure (CPAP) and intermittent positive pressure ventilation (IPPV) in patients with flail chest. Emerg Med J. 2005;22:325.
- Walters MK, Farhat J, Bischoff J, Foss M, Evans C. Ketamine as an Analgesic Adjuvant in Adult Trauma Intensive Care Unit Patients With Rib Fracture. Ann Pharmacother. 2018;52:849–54.
- Carver TW, Kugler NW, Juul J, Peppard WJ, Drescher KM, Somberg LB, et al. Ketamine infusion for pain control in adult patients with multiple rib fractures. J Trauma Acute Care Surg. 2019;86:181–8.
- Kugler NW, Carver TW, Juul J, Peppard WJ, Boyle K, Drescher KM, et al. Ketamine infusion for pain control in elderly patients with multiple rib fractures: Results of a randomized controlled trial. J Trauma Acute Care Surg. 2019;87:1181–8.
- Hashemzadeh S, Hashemzadeh K, Hosseinzadeh H, Maleki RA, Golzari SEJ, Golzari S. Comparison thoracic epidural and intercostal block to improve ventilation parameters and reduce pain in patients with multiple rib fractures. J Cardiovasc Thorac Res. 2011;3:87–91.
- Britt T, Sturm R, Ricardi R, Labond V. Comparative evaluation of continuous intercostal nerve block or epidural analgesia on the rate of respiratory complications, intensive care unit, and hospital stay following traumatic rib fractures: a retrospective review. Local Reg Anesth. 2015; 8:79–84.
- Agamohammdi D, Montazer M, Hoseini M, Haghdoost M, Farzin H. A Comparison of Continuous Thoracic Epidural Analgesia with Bupivacaine Versus Bupivacaine and Dexmedetomidine for Pain Control in Patients with Multiple Rib Fractures. Anesth Pain Med. 2018;8:1-7.
- Luchette FA, Radafshar SM, Kaiser R, Flynn W, Hassett JM. Prospective Evaluation of Epidural versus Intrapleural Catheters for Analgesia in Chest Wall Trauma. J Trauma. 1994;36:865–70.
- Malekpour M, Hashmi A, Dove J, Torres D, Wild J. Analgesic Choice in Management of Rib Fractures. Anesth Analgesia. 2017;124:1906–11.
- Mohta M, Verma P, Saxena AKr, Sethi AK, Tyagi A, Girotra G. Prospective, Randomized Comparison of Continuous Thoracic Epidural and Thoracic Paravertebral Infusion in Patients With Unilateral Multiple Fractured Ribs—A Pilot Study. J Trauma. 2009;66:1096–101.
- Mackersie RC, Shackford SR, Hoyt DB, Karagianes TG. Continuous epidural fentanyl analgesia: ventilatory function improvement with routine use in treatment of blunt chest injury. J Trauma. 1987;27:1207–12.
- Dittmann M, Ferstl A, Wolff G. Epidural analgesia for the treatment of multiple rib fractures. Eur J Inten Care Med. 1975;1:71–5.
- Govindarajan R, Bakalova T, Michael R, Abadir AR. Epidural buprenorphine in management of pain in multiple rib fractures. Acta Anaesth Scand. 2002;46:660–5.
- Rankin APN, Comber REH. Management of Fifty Cases of Chest Injury with a Regimen of Epidural Bupivacaine and Morphine. Anaesth Intens Care. 1984;12:311–4.
- Baker EJ, Lee GA. A Retrospective Observational Study Examining the Effect of Thoracic Epidural and Patient Controlled Analgesia on Short-term Outcomes in Blunt Thoracic Trauma Injuries. Medicine (Baltimore). 2016;95:1-8.
- Gage A, Rivara F, Wang J, Jurkovich GJ, Arbabi S. The effect of epidural placement in patients after blunt thoracic trauma. J Trauma Acute Care Surg. 2014;76:39–46.
- Jensen CD, Stark JT, Jacobson LL, Powers JM, Joseph MF, Kinsella-Shaw JM, et al. Improved Outcomes Associated with the Liberal Use of Thoracic Epidural Analgesia in Patients with Rib Fractures. Pain Med. 2016;18:1787–94.
- Kieninger AN, Bair HA, Bendick PJ, Howells GA. Epidural versus intravenous pain control in elderly patients with rib fractures. Am J Surg. 2005;189:327–30.
- Lynch N, Salottolo K, Foster K, Orlando A, Koola C, Portillo V, et al. Comparative effectiveness analysis of two regional analgesia techniques for the pain management of isolated multiple rib fractures. J Pain Res. 2019;12:1701–8.
- McKendy KM, Lee LF, Boulva K, Deckelbaum DL, Mulder DS, Razek TS, et al. Epidural analgesia for traumatic rib fractures is associated with worse outcomes: a matched analysis. J Surg Res. 2017;214:117–23.
- O’Connell KM, Quistberg DA, Tessler R, Robinson BRH, Cuschieri J, Maier RV, et al. Decreased Risk of Delirium With Use of Regional Analgesia in Geriatric Trauma Patients With Multiple Rib Fractures. Ann Surg. 2018;268:534–40.
- Wisner DH. A Stepwise Logistic Regression Analysis of Factors Affecting Morbidity and Mortality After Thoracic Trauma. J Trauma. 1990;30:799–805.
- Wu CL, Jani ND, Perkins FM, Barquist E. Thoracic Epidural Analgesia versus Intravenous Patient-Controlled Analgesia for the Treatment of Rib Fracture Pain after Motor Vehicle Crash. J Trauma. 1999;47:564–7.
- Yeh DD, Kutcher ME, Knudson MM, Tang JF. Epidural analgesia for blunt thoracic injury—Which patients benefit most? Inj. 2012;43:1667–71.
- Zaw AA, Murry J, Hoang D, Chen K, Louy C, Bloom MB, et al. Epidural Analgesia after Rib Fractures. Am Surg. 2015;81:950–4.
- Ahmed SM, Athar M, Ali S, Doley K, Siddiqi OA, Usmani H. Acute pain services in flail chest-a prospective randomized trial of epidural versus parenteral analgesia in mechanically ventilated ICU patients. Egypt J Anaesth. 2015;31:327–30.
- Bulger EM, Edwards T, Klotz P, Jurkovich GJ. Epidural analgesia improves outcome after multiple rib fractures. Surgery. 2004;136:426–30.
- MacKersie RC, Karagienes TG, Hoyt DB, Davis JW. Prospective Evaluation of Epidural and Intravenous Administration of Fentanyl for Pain Control and Restoration of Ventilatory Function Following Multiple Rib Fractures. J Trauma. 1991;31:443–51.
- Ullman DA, Fortune JB, Greenhouse BB, Wimpy RE, Kennedy TM. The treatment of patients with multiple rib fractures using continuous thoracic epidural narcotic infusion. Region Anesth. 1989;14:43–7.
- Haenel JB, Moore FA, Moore EE, Sauaia A, Read RA, Burch JM. Extrapleural Bupivacaine for Amelioration of Multiple Rib Fracture Pain. J Trauma. 1995;38:22-27.
- Karmakar MK, Critchley LAH, Ho AM-H, Gin T, Lee TW, Yim APC. Continuous Thoracic Paravertebral Infusion of Bupivacaine for Pain Management in Patients With Multiple Fractured Ribs. Chest. 2003;123:424–31.
- Truitt MS, Murry J, Amos J, Lorenzo M, Mangram A, Dunn E, et al. Continuous Intercostal Nerve Blockade for Rib Fractures: Ready for Primetime? J Trauma. 2011;71:1548–52.
- Osinowo OA, Zahrani M, Softah A. Effect of Intercostal Nerve Block with 0.5% Bupivacaine on Peak Expiratory Flow Rate and Arterial Oxygen Saturation in Rib Fractures. J Trauma. 2004;56:345–7.
- Womack J, Pearson JD, Walker IA, Stephens NM, Goodman BA. Safety, complications and clinical outcome after ultrasound-guided paravertebral catheter insertion for rib fracture analgesia: a single-centre retrospective observational study. Anaesth. 2019;74:594–601.
- Hernandez N, Haan J de, Clendeninn D, Meyer DE, Ghebremichael S, Artime C, et al. Impact of serratus plane block on pain scores and incentive spirometry volumes after chest trauma. Local Regional Anesth. 2019;12:59–66.
- Adhikary SD, Liu WM, Fuller E, Cruz-Eng H, Chin KJ. The effect of erector spinae plane block on respiratory and analgesic outcomes in multiple rib fractures: a retrospective cohort study. Anaesth. 2019;74:585–93.
- Yeying G, Liyong Y, Yuebo C, Yu Z, Guangao Y, Weihu M, et al. Thoracic paravertebral block versus intravenous patient-controlled analgesia for pain treatment in patients with multiple rib fractures. J Int Med Res. 2017;45:2085–91.
- Gabram SGA, Schwartz RJ, Jacobs LM, Lawrence D, Murphy MA, Morrow JS, et al. Clinical management of blunt trauma patients with unilateral rib fractures: A randomized trial. World J Surg. 1995;19:388–93.
- Tignanelli CJ, Rix A, Napolitano LM, Hemmila MR, Ma S, Kummerfeld E. Association Between Adherence to Evidence-Based Practices for Treatment of Patients With Traumatic Rib Fractures and Mortality Rates Among US Trauma Centers. JAMA Netw Open. 2020;3:1-14.
- Joseph B, Pandit V, Zangbar B, Kulvatunyou N, Hashmi A, Green DJ, et al. Superiority of Frailty Over Age in Predicting Outcomes Among Geriatric Trauma Patients. JAMA Surg. 2014;149:766–72.
- Brasel KJ, Moore EE, Albrecht RA, deMoya M, Schreiber M, Karmy-Jones R, et al. Western Trauma Association Critical Decisions in Trauma. J Trauma Acute Care Surg. 2017;82:200–3.
- Galvagno SM, Smith CE, Varon AJ, Hasenboehler EA, Sultan S, Shaefer G, et al. Pain management for blunt thoracic trauma. J Trauma Acute Care Surg. 2016;81:936–51.
- Pierre E, Martin P, Frohock J, Varon A, Barquist E. Lumbar epidural morphine versus patient-controlled analgesia morphine in patients with multiple rib fractures. Anesthesiology. 2005;103:A289.
- Moon MR, Luchette FA, Gibson SW, Crews J, Sudarshan G, Hurst JM, et al. Prospective, Randomized Comparison of Epidural Versus Parenteral Opioid Analgesia in Thoracic Trauma. Ann Surg. 1999;229:684.